Recap of our September 10, 2014 Meeting
at Wilkes-Barre General Hospital
Hosted by: Maria Mattei
Top Tips for Managing Your POC Program
I A Dialogue on Preparing for IQCPs
Top Tips for Managing Your POC Program
Kim Skala, MT(ASCP), IL
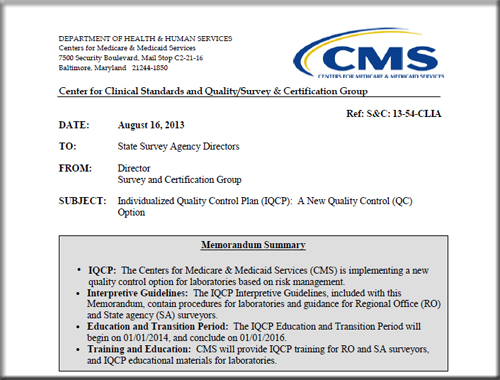
Individualized Quality Control Plan (IQCP): A New Quality Control (QC) Option
IQCP: The Centers for Medicare & Medicaid Services (CMS) is implementing a new quality control option for laboratories based on risk management.
•
Interpretive Guidelines: The IQCP Interpretive Guidelines, included with this Memorandum, contain procedures for laboratories and guidance for Regional Office (RO) and State agency (SA) surveyors.•
Education and Transition Period: The IQCP Education and Transition Period will begin on 01/01/2014, and conclude on 01/01/2016.•
Training and Education: CMS will provide IQCP training for RO and SA surveyors, and IQCP educational materials for laboratories.
A Dialogue on Preparing for IQCPs
Thomas I. Koshy, Ph.D., Alere
Return to PointofCare.net Home Page • Last updated: 10/06/2014
Questions: editor@pointofcare.net