PixCell Medical Inc.
825 Delaware Ave.
Suite 304
Longmont, CO 80501
Phone: 720-715-7215
customerservice@pixcell-medical.com |

|
COMPANY INFORMATION
At PixCell Medical, we believe that simply having blood diagnostic information is not enough. Meaningful action is needed to create a real impact, in real life. That’s why we have designed revolutionary rapid complete blood count testing diagnostics that are specifically crafted to equip practitioners and patients with the insight they need to take quick action when it matters most.
PixCell is led by an experienced management team with an accumulated 50 years of experience in medical product commercialization. Our team consists of a highly innovative and multi-disciplinary group of scientists, engineers, and business leaders. PixCell Medical’s US corporate headquarters are located in Longmont, Colorado.
Products on this page include: HEMOSCREEN | HEMOSCREEN TEST KIT | HEMOSCREEN SOFTWARE |
|
HEMOSCREEN
| CLIA Classification: |
Moderately Complex |
 |
| Size and weight: |
11.8 in (height), 6.9 in (width), 10.2 in (depth); 12.1 lbs |
| Specifications: |
2 USB ports & Ethernet connection |
| Data transfer capability: |
Ethernet / Bi-directional |
| Features: |
- Comprehensive: Measures 20 standard CBC parameters, including 5-part leukocyte differential.
- Simple: Uses a disposable “lab-on-a-cartridge” that includes all necessary reagents.
- Accurate: Accuracy and precision of advanced central lab analyzers.
- Rapid: 5 minutes from test to result.
- Robust: No maintenance, no calibration, liquid-free design.
|
| Test Menu: |
| CBC With 5-Part Differential: |
CBC Without Differential: |
- WBC
- NEU# & NEU%
- LYM# & LYM%
- MON# & MON%
- EOS# & EOS%
- BAS# & BAS%
- RBC
- HGB
- HCT
- MCV
- MCH
- MCHC
- RDW-CV
- PLT
- MPV
- Including flagging of IG, nRBC, blasts and atypical lymphocytes
|
- WBC
- RBC
- HGB
- HCT
- MCV
- MCH
- MCHC
- RDW-CV
- PLT
- MPV
- Including flagging of IG, nRBC, blasts and atypical lymphocytes
|
| CPT code for each analyte: 85025 |
CPT code for each analyte: 85027 |
|
| Test Methodology: |
HemoScreen uses a patented technique called viscoelastic focusing, which causes the cells to perfectly align into a single plane. High resolution microscopic images are taken of the flowing cells. Each image is then analyzed using machine vision AI algorithms, and the various cell types are differentiated and counted. WBCs are stained prior to analysis to enable differentiation between their sub-types and abnormal cells. HGB is calculated based on the optical density measured on individual, intact cells. |
| Units in which the test result is reported: |
- WBC: 10^3/µL
- RBC: 10^6/µL
- HGB: g/dL
- HCT: %
- MCV: fL
- MCHC: g/dL
- RDW: %
- PLT: 10^3/µL
- MPV: fL
- NEU#, LYM#, MON#, EOS#, BAS#: 10^3/µL
- NEU%, LYM%, MON%, EOS%, BAS%: %
|
| MSDS's associated with this product etc.: |
N/A |
|
|
HEMOSCREEN TEST KIT
| CLIA Classification: |
Moderately complex |
 |
| Analytes performed on this device |
CBC With Differential, CBC Without Differential |
| CPT Code associated with each analyte: |
85025 (CBC With Differential), 85027 (CBC Without Differential) |
| Test methodology: |
HemoScreen’s disposable cartridge includes all necessary reagents, automatically preparing the sample and eliminating potential pre-analytical errors. The sample is precisely dosed based on capillary action and is automatically injected into the reagent chambers within the cartridge. Two 20 µL samples are mixed and incubated in parallel chambers. The samples undergo treatment and staining, and flow at different stages into a microfluidic measurement chamber for analysis and enumeration. The cartridge removes cross-contamination risk and makes sample preparation user independent. |
| Sample volume: |
CBC + 5-part Diff: 40 µL |
| Time to result: |
5 minutes for CBC + 5-Part Diff. / 3 minutes for CBC only |
| Units in which the test is reported: |
- WBC: 10^3/µL
- RBC: 10^6/µL
- HGB: g/dL
- HCT: %
- MCV: fL
- MCHC: g/dL
- RDW: %
- PLT: 10^3/µL
- MPV: fL
- NEU#, LYM#, MON#, EOS#, BAS#: 10^3/µL
- NEU%, LYM%, MON%, EOS%, BAS%: %
|
| MSDS's associated with the use of this product: |
This product consists of three mixtures of ingredients. Each mixture is contained in its own housing that are mounted on the cartridge. The ingredients in each housing (such as dyes, detergents, buffer solutions and preservatives) are in concentrations not associated with human or environmental toxicity. MSDS for HemoScreen CBC Cartridge available upon request. |
|
|
HEMOSCREEN SOFTWARE
 |
 |
 |
 |
 |
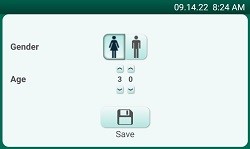
| Home Screen |
Demographic Screen |
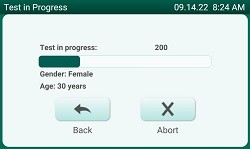
Test in Progress Screen |
Test Results Screen |
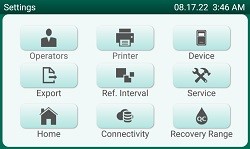
Settings Screen |
| Current version # availability: |
2.2.1 |
| Included download capability: |
Upgrade software via LIS or USB |
| Operating system capability: |
Windows 10 |
| Administration features: |
Customizable list and authorization of operators |
| User features: |
- LCD display and touch panel
- Optional barcode reader & direct thermal printer configuration
- Extensive customizable settings menu
|
| QC features: |
- PIX002 PIX-CBC 3-levels liquid control
- Target values imported via 2D barcode reader or USB
- Can be ordered from PixCell Medical Inc
|
| Instrument Management features: |
Three operator authentication levels of device management |
| Inventory Control features: |
Import and storage of control reference ranges |
| Reporting features: |
Reports CBC results on LCD display with additional tab for WBC Differential |
| Export capability: |
Ethernet & USB connection |
| LIS Interface capability: |
HL7 (Unidirectional), POCT1-A (Unidirectional & Bi-directional) |
|
| |
|

