Tight and terrible: Lab leaders on budgets and staffing
CAP TODAY, December 2021
The staffing crisis lives on, despite labs having plans of all kinds in place to alleviate the shortage. “It’s the only thing we’re talking about,” Ochsner Health’s Greg Sossaman, MD, said on Nov. 2 when members of the Compass Group met by Zoom. SARS-CoV-2 testing and test supplies and vaccination are “taking a back seat” to staffing, he said. CAP TODAY publisher Bob McGonnagle led the roundtable last month, when COVID-19 positivity rates were up in some areas and down in others. Here is what Dr. Sossaman and other lab leaders had to say. The Compass Group is an organization of not-for-profit IDN system lab leaders who collaborate to identify and share best practices and strategies.
Read more >
Saving Your Sanity With POCT Connectivity
November 2021, Clinical Laboratory News, By Kerstin Halverson, BA, MS Point-of-care testing (POCT) is designed to place devices at or near the bedside and provide results as quickly as possible. However, the distance of these devices from the lab presents a dilemma for getting results into the patient’s chart as quickly as possible. Various connectivity solutions have evolved to deal with this problem.
Some manufacturers’ software enables the result to pass from the device to the lab information system (LIS) and then into the hospital information system (HIS) or electronic medical record (EMR). There are also vendor-neutral middleware solutions that can connect many POC devices, regardless of manufacturer.
Read more >
New hope for lab data interoperabilityCAP Today, November 2021, By Anne Paxton
November 2021—Interoperability, a problem of long standing in health care, has a new push and new prospects. Interoperability has become a front-burner issue because it has become increasingly urgent to bring the standardized communication of health care data up to speed. Since the 2009 Affordable Care Act, significant resources have been directed to bringing widespread use of electronic health record systems. But interoperability among those EHRs has been held up by the lack of mechanisms and standards to ensure interoperability of laboratory data, which has drawn special concern during the pandemic.
An initiative led by federal agencies could turn that shortage of standards for laboratory data around. The key is a public/private initiative called SHIELD (Systemic Harmonization and Interoperability Enhancement for Laboratory Data), launched in 2016 by the Food and Drug Administration. Starting early in 2022, the profile of lab data interoperability should rise as SHIELD takes major public steps forward.
Read more >
Rapid Point-of-Care Antigen Assay Could Aid Hospital Infection Control
October 2021 | Clinical Laboratory News
A new assay might detect SARS-CoV-2 antigens with enough sensitivity and specificity to inform infection control measures and potentially inform novel, point-of-care testing methods, according to a recent Clinical Chemistry paper. Many nucleic acid-based methods are sensitive enough to detect acute COVID-19 infections. However, persistent nucleic acid positivity after symptom resolution and disease recovery complicates infection control measures. This is true especially in immunocompromised patients who show long periods of nucleic acid positivity and have diverse presentations.
Read more >
AST and safety at core of microbiology checklist changes
October 2021 | CAP Today | Valerie Neff Newitt
By Jan. 1, 2024, laboratories must use current breakpoints to interpret antimicrobial minimum inhibitory concentration and disk diffusion test results, according to a new requirement in the latest edition of the CAP Accreditation Programs microbiology checklist, released Sept. 22. The same requirement calls for laboratories to implement new breakpoints within three years of the official publication date of the updated breakpoint.
“That is going to be a challenge and real work for a lot of laboratories,” Sheldon Campbell, MD, PhD, a member of the CAP Checklists Committee, says of the new requirement (MIC.11385 Current Antimicrobial Susceptibility Test Interpretation Breakpoints). His advice to laboratories: “Start thinking now about how you are going to accomplish that.”
The new requirement is one of several changes to the 2021 microbiology checklist. Those changes were made for three main reasons, the first having to do with clarity.
Read more >
The Value of Molecular POCT for Managing Infectious Diseases
By Kim Futrell, MT (ASCP), MSHI, Aug 25th, 2021, MLO
Point-of-care testing (POCT) technology continues to advance at a fast pace, making it easier to shrink complex testing platforms into point-of-care (POC) devices. Though not without its challenges, testing at a molecular level far surpasses the accuracy of other POCT methodologies. Molecular POCT devices allow quick, easy-to-use testing in near-patient scenarios where a rapid diagnosis can be the difference between treating one patient and treating a multitude of patients who become infected because the initial diagnosis is delayed.
Molecular testing is a broad term that refers to the detection and/or quantification of specific DNA or RNA sequences in a specimen. Molecular tests are used to detect microorganisms, look for genetic mutations associated with certain diseases and cancers, perform paternity tests, and much more. Highly complex molecular testing is performed in molecular or microbiology specialty laboratories by trained laboratory professionals.
Read more >
FDA approves OTC and POC COVID-19 tests
MLO - April 2021 - The U.S. Food and Drug Administration (FDA) approved amended emergency use authorization (EUA) requests for multiple tests this week, expanding over-the-counter and point-of-care testing options for COVID-19, the agency said in a news release. “The addition of the OTC and POC tests for screening will give schools, workplaces, communities and others several options for serial screening tests that are accurate and reliable,” the FDA said. The authorizations the FDA approved are:
- Quidel QuickVue At-Home OTC COVID-19 test - authorized for OTC at-home serial screening
- Abbott BinaxNOW COVID-19 Antigen Self Test – authorized for OTC at-home serial screening
- Abbott BinaxNOW COVID-19 Ag Card 2 Home Test – authorized for OTC at-home serial screening with telehealth
- Abbott BinaxNOW COVID-19 Ag 2 Card – authorized for POC serial screening without a prescription
- BD Veritor System for Rapid Detection of SARS-CoV-2 – authorized for POC serial screening with a prescription
Read more >
Coronavirus: Guidance for Better Mental Health
During the COVID-19 pandemic, there will be a lot of information about the virus and its effects on mental health. That’s because coronavirus and the social, financial and psychological implications it carries can seriously impact one’s mental wellbeing. Government legislation, mass media coverage, and the increasing global death toll will cause a lot of stress, especially for the older population, children, and people with a history of mental health problems.
It’s of the utmost importance that we try to remain as composed as we can during this time. The fear and anxiety that is gripping the nation are as contagious, if not more so than the illness itself. Learn more >
Know the Curves Guide to COVID-19 Testing
Educational guide helps anyone understand COVID-19 testing
Testing is an important tool in the fight against COVID-19. But many people are still confused about the different types of tests that are available. This simple, non-commercial, guide is written in clear language so anyone can understand:
- The difference between viral RNA, antigen, and antibody testing
- Why it’s important to get the right test at the right time
- What a positive result means
- When people are most contagious
- And more
Feel free to forward this to patients, friends, family, and anyone else who might be interested. Download the PDF here
Checklist, CLIA line up on COVID-19 Reporting
CAP Today, November 2020, By Anne Paxton - It’s been well understood since the Ten Commandments that rules that appear simple in theory can be fiendishly complex or even impossible to execute. The pandemic is providing a perfect example of that in the laboratory world, but with added twists, at least for now: It may not be clear which rules are mandatory, desirable, or optional—and those aren’t the only sources of confusion. Since March, much attention at the federal level has been focused on clear standards for reporting results of SARS-CoV-2 testing. But many fear that new rules to standardize reporting could require hammering a multitude of diverse square pegs into round holes. At present, labs will be penalized only if they fail to report both positive and negative test results to their state and local public health authorities. While the June 4 guidance is required, the Centers for Medicare and Medicaid Services has said it will enforce only a few key aspects. Read more >
At POC and in the Lab, 2 new checks on SARS-CoV-2 testing
CAP Today, November 2020, By Valerie Neff Newitt - The CAP released in September its proficiency testing program for SARS-CoV-2 antigen testing, with the first shipment to laboratories set for Nov. 30. It also introduced recently a Quality Cross Check program that makes it possible for labs performing nucleic acid amplification testing for SARS-CoV-2 to monitor performance across multiple instruments, in compliance with the CMS directive prohibiting proficiency testing on multiple instruments. “Even with its limitations, antigen testing could have a role in a well-designed testing strategy, but nucleic acid testing remains the standard of care for a definitive diagnosis,” says Daniel D. Rhoads, MD, member of the CAP Microbiology Committee, which helped to develop the proficiency test. With laboratory medicine having “pushed to the forefront of news” during the pandemic, “it’s critically important that we are performing well in all testing formats and meeting the need in this time of crisis,” says Dr. Rhoads, assistant professor and section head of microbiology at the Cleveland Clinic.
Read more >
UMASS' JoAnn Crain Named 2020 POCC of the Year!
As the Point of Care Manager, JoAnn Crain oversees and coordinates the POCT for the entire UMass Memorial Healthcare System, including multiple member hospitals and several remote locations. Currently, she manages the
POCT under 10 CLIA licenses with over 100 departments in various hospitals and clinics. JoAnn oversees training and competencies of thousands of clinical POCT users and reviews all Quality Control, Quality Assurance records and all necessary testing validations. The Point-of-Care Testing (POCT) Coordinator Award is given annually to recognize outstanding achievements in the POCT field by persons who are primarily responsible for a given institution’s POCT program. The award selection is made by the Awards Committee of the AACC Critical and Point-of-Care Testing (CPOCT) Division and is based on the extent of the nominee’s responsibilities and accomplishments, particularly the impact this person has made in improving the quality of the POCT program at their facility. Read more >
John Peterson, PhD Receives AACC Outstanding Contributions to POCT Award!
John Petersen has trained professional clinical laboratory scientists and taught critical and point of care testing at the undergraduate, postgraduate, and practicing level. He has conducted meritorious scientific and educational research, and made contributions to the critical and point of care testing field at multiple professional organizations. Dr. Petersen has actively participated in AACC to support point of care programs and educational activities. He has served on the Critical and Point of Care Testing Division’s nomination committee, was chair of the Awards committee, a Member at Large, and also Treasurer. The Outstanding Contributions to Point of Care Testing Award recognizes an individual who has made an outstanding contribution in the field of critical and point of care testing. This may be for a single outstanding achievement, or for a body of accomplishments that have broadly advanced the field of point-of-care testing. Such accomplishments may include, but are not limited to, the training of professional clinical laboratory scientists; the teaching and integration of critical and point of care testing at the undergraduate, postgraduate, and practicing level; meritorious scientific, educational and research contributions; and contributions through service to critical and point of care testing. Read more >
Flu Mounts COVID's Bustling Stage
CAP Today, October 2020 - Barely a half year into the pandemic’s presence in the United States, history has already begun pressing down on SARS-CoV-2 testing. Like an actor playing Hamlet, it’s been difficult not to feel the burden of
past performances when preparing for the months ahead. Now, at the start of fall, that also means readying for the return of influenza. Here, even longer experience has shown that each new season is, indeed, a new season. Read more >
States Scaling Up Rapid COVID-19 Tests
MLO, October, 2020 - State governments are distributing Abbott BinaxNOW COVID-19 tests to numerous types of institutions, the Department of Health and Human Services (HHS), which purchased the tests, said in a press release.Of the states that have provided preliminary reports, use of the BinaxNOW allocations are largely being deployed to local health departments, K-12 schools and universities, nursing homes, hospitals and correctional facilities, HHS said. Other states, however, have additional priorities. For example, Alaska is sending tests to oil drilling sites; Mississippi and other states to veterans’ homes; Nevada to tribal health clinics; and Colorado to local public health agencies to test homeless populations.HHS purchased 150 million Abbott BinaxNOW rapid tests from Abbott in August... Read more >
Managing Point-of-Care Testing During COVID-19: What We’ve Learned
Kathleen David, MT (ASCP), Manager, Point-of-Care Testing, TriCore Reference Laboratories
What have we learned from COVID-19? The bigger questions may be, why was there so much to learn? Why weren’t we, in the pointof-care testing (POCT) community, better prepared, we who have been through the Zika and Ebola viruses, as well as Hurricane Katrina and other health crises? The truth is, there is no one-size-fits-all when it comes to disaster planning. COVID-19 is not Zika, and Zika was not Ebola. It is also true that there is a dearth of published literature or other trusted resources, specifically addressing disaster planning for POCT.
The intent of this article is not to provide answers, but to start a conversation in the POCT community. Asking ourselves better questions now can help us be better prepared for future pandemics and crises. We know they will come, as will further waves of COVID-19, which threatens to make the coming flu season particularly challenging. Read the article>
CLSI Webinar: Thanks to CLSI, a Zoom Webinar that took place on Wednesday, September 9, was recorded and available
for playback on their eLearning platform. Click here or on the image to the right to register for the webinar. There is no cost to register, but if you don’t already have a CLSI account, you’ll need to create one by registering your email address and creating a password.
As the Pandemic Meets Flu Season, Labs Turn to Rapid Molecular Testing
by Sherry A. Dunbar, MBA, PhD., MLO, August 2020
Bracing for flu season is challenging for most clinical labs even in the best of times. With the COVID-19 pandemic, though, laboratories will face an unprecedented respiratory testing situation this fall and winter. Rapid molecular tests and flexible platforms that allow for multiplexing several pathogens in a single assay will be an essential tool for dealing with the potential crisis that lies ahead of us and should help to ease the supply chain stress associated with dramatically higher testing rates. Read the article >
The Laboratory Tests of a Pandemic Summer
Karen Titus, CAP Today, August 2020
In March, the COVID-19 pandemic came in like a lion—and has yet to leave, like a lamb or anything else. Instead, it roared through April and May in early hot spots like New York City and New Orleans. As lockdowns took hold, the cautious hope was that by summer the virus would be tamed (if not simply go away “like a miracle” or “as the heat comes in,” per several infamous predictions), giving health care providers a chance to exhale before a likely second wave in the fall. Instead, June and July saw other cities and states hit hard in turn, while many places that appeared to have flattened the curve were starting to see concerning upticks in cases. And rather than planning for a return of the virus later in the year, laboratories are now talking about SARS-CoV-2 as an ongoing presence. Read more >
HHS CARES Act Guidance for Labs
The U.S. Department of Health and Human Services (HHS) released new guidance related to laboratory result reporting specified in the Coronavirus Aid, Relief, and Economic Security (CARES) Act. The new guidance covers entities required to report, methods for submission, required data elements, data reporting and transmission requirements, and recommendations for capturing laboratory data in electronic health records. The new guidance is now posted on CDC’s
How to Report COVID-19 Laboratory Data web page.
A Panel’s Take on Instruments, Connectivity, COVID
CAP Today, July 2020
Has the pandemic changed your thinking or that of your customers? That’s one of the questions CAP TODAY
publisher Bob McGonnagle put to seven representatives of five companies and two other panelists in a roundtable on chemistry/immunoassay analyzers and testing. But first up were other topics: scalability, connectivity, standardizing platforms across health systems, consistent sourcing of antibodies, and open automation.
The panelists were Gyorgy Abel, MD, PhD, of Lahey Hospital and Medical Center; David Grenache, PhD, D(ABCC), of TriCore Reference Laboratories; Brittany Greiner of Roche Diagnostics; Denise Pastore of Siemens Healthineers; John Naizer, BSc, MSc, of Randox; Timea Zsiray and Sean Roberts of Beckman Coulter; and Chad Meyers and Jeffrey Watson, MT(ASCP), MBA, of Sunquest Information Systems. Read what they had to say.
AACC releases guidance document on using POC tests to improve patient care
MLO, June 2020
AACC has issued a new guidance document detailing best practices that hospitals and other healthcare institutions should follow when running a point-of-care testing program. As point-of-care tests emerge for more and more conditions—including COVID-19—the guidance emphasizes that it is essential for laboratory professionals and clinicians to collaborate on point-of-care testing programs to ensure this testing benefits patients.Point-of-care tests are clinical tests that can be performed... Read more >
A Lab World Embroiled in Pandemic
CAP Today, May 2020, By Karen Titus
Along with SARS-CoV-2, clinical laboratory testing has been hiding in plain sight far longer than many people realize.
But it took the novel coronavirus (which, frankly, hardly feels novel anymore) to make that clear to the rest of the world.
As the COVID-19 pandemic spread across the globe, laboratory testing crashed the news cycle. National leaders sought to reassure citizens by promising millions of test kits. Economies shattered—and have since sought to return to life—based on
testing availability. Ordinary people woke before dawn to wait in lines at drive-through testing sites—often to be turned away when supplies ran out. Emergency use authorization became a common phrase. And behind every heartbreaking photo from an emergency department or ICU lingered an unnerving thought: We don’t have tests. Read more >
What’s Next on the Point-of-Care Testing Menu?
From SARS-CoV-2 to cardiac biomarkers, a steady stream of IVD innovation shows no signs of letting up
By Kimberly Scott, MAY 2020, Clinical Laboratory News
While point-of-care (POC) testing in recent years has drastically altered how patients are treated for conditions such as diabetes, HIV, and cardiovascular disease, new advancements on the horizon are expected to vastly improve near-patient treatment for strokes, infectious diseases, and cancer, according to experts.
In vitro diagnostics (IVD) companies are working to improve specificity and sensitivity of devices so that testing can be done on smaller specimen samples, said Nick Collier, PhD, chief technology officer for Sagentia Medical, a contract research organization based in the U.K. “There is a lot of interest in reducing sample size—using capillary blood samples to do testing, for example.”
While there are more than 100 POC tests available in the U.S., not all are widely implemented, such as tests for proteins in blood used for cancer diagnosis, noted Kathleen David, MT(ASCP), POC testing manager for TriCore Reference Laboratories in Albuquerque, New Mexico. “Availability is one thing. Then there’s acceptance and implementation,” she said. “Some of these we will see in cutting-edge places in the next couple of years...' Read more >
AACC Reschedules 2020 Annual Meeting & Clinical Lab Expo to December
The ongoing spread of COVID-19 is a serious concern and as its global impact grows daily, AACC’s priority is always the health and safety of our community. AACC’s leadership has been carefully monitoring the outbreak and has been consulting members, staff, industry, and other experts.
Based on input from all stakeholder groups, and in close collaboration with host city officials, the organization is pleased to announce that AACC will be able to preserve the complete Annual Scientific Meeting & Clinical Lab Expo experience to which its members, exhibitors, and the entire laboratory medicine community have been looking forward. The 2020 AACC Annual Scientific Meeting & Clinical Lab Expo will now be held December 13 – 17, 2020 at McCormick Place in Chicago, IL. Read more >
Congratulations to Lenox Hill Hospital as the winner of MLO’s 2020 Lab of the Year Award!
Located in New York City’s prestigious Upper East Side, Lenox Hill Hospital opened its doors to the community in 1865 and continues to serve approximately 325,000 patients per year through those same doors. Earning its reputation over the years as a teaching hospital, the 632-bed Lenox Hill Hospital has provided care to both celebrity and layperson alike, with the same attention to treating a wide range of medical conditions and diseases. Read more >
Runners Up - Norman Regional Hospital | Mary Lanning Healthcare
Hospitals to report COVID-19 test data daily to the federal government
The Centers for Medicare & Medicaid Services (CMS) sent a letter to the nation’s hospitals, detailing how they should report data on tests they are performing to detect COVID-19, the agency said in a press release. The letter was sent on behalf of Vice President Mike Pence. Specifically, the federal government wants hospitals to report daily data on COVID-19 test results conducted at in-house labs to both the Department of Health and Human Services (HHS) and the Centers for Disease Control and Prevention (CDC) National Healthcare Safety Network. CMS said the data should
not include personal identifying information to protect patients’ privacy. Read more >
Staying Connected with POCT
Use online resources to keep up with what's happening in POCT
While every news bureau will keep you updated on the latest COVID-19 happenings, POC resources are also available that will enable you to stay connected with what is happening in our community.
POC Listservs
Listervs are a great way to communicate with your POC peers and if you are not part of one, or many, we encourage you to join. Examples include the POCT Listerv, AACC LIstserv and the AACC Artery can be found in the column to the right.
POC Webinars
Whitehat Communications will continue to host POC Group webinars through the support of POC vendors. You can visit the Whitehat website or our calendar pages for upcoming webinars. POC vendors such as Siemens, Orchard, Fisher and others also have webinars on their websites that you can listen to. Click here for a Google search page.Others, such as Abbott Rapid Diagnostics Informatics / RALS hold monthly sessions that you join.
POC Publications
While publications will most likely continue to print and distribute the journals, you can also go online to latest from CAP Today,Medical Laboratory Observer (MLO), Clinical Laboratory News and others. Visit our POC publications page to access their websites.
Abbott launches molecular point-of-care test novel coronavirus in as little as 5 minutes
ID NOW™ COVID-19 tests available to HCPs in urgent care settings in the U.S
March 27, 2020 - Abbott announced today that the U.S. Food and Drug Administration (FDA) has issued Emergency Use Authorization (EUA) for the fastest available molecular point-of-care test for the detection of novel coronavirus
(COVID-19), delivering positive results in as little as five minutes and negative results in 13 minutes. The test will run on the company's ID NOW™ platform, providing rapid results in a wide range of healthcare settings such as physicians' offices, urgent care clinics and hospital emergency departments. The ID NOW platform is small, lightweight (6.6 pounds) and portable (the size of a small toaster), and uses molecular technology, which is valued by clinicians and the scientific community for its high degree of accuracy. ID NOW is already the most widely available molecular point-of-care testing platform in the U.S. today. Read more >
FDA authorizes first rapid ‘point-of-care’ test for coronavirus
The test, to be used in hospitals, will deliver results in 45 minutes
The Food and Drug Administration approved the first coronavirus test that can be conducted entirely at the point of care for a patient — and deliver results in 45 minutes. The FDA granted “emergency use authorization” to Cepheid, a California company that makes a rapid molecular test for the coronavirus. The turnaround time for Cepheid’s product is far shorter than for the tests being used, which are typically sent to centralized labs that may not return results for days. The FDA authorization is for use in “patient care settings,” including doctors’ offices, but the test initially will be used primarily by hospitals and emergency departments, the company said.
As covid-19 cases proliferate, fears are growing that hospitals will become overwhelmed by patients seeking tests or care. David Persing, Cepheid’s chief medical and technology officer, said in an interview that the test will “help alleviate the pressure” on health-care facilities by helping doctors find out quickly whether a patient has the disease and select the appropriate treatment. “This is not a test for the worried well,” he said, but rather a tool for doctors to quickly assess patients suspected of having covid-19. The specimen can be collected either by a nasal swab or by a saline wash using a small catheter. Neither is particularly comfortable, but the advantage of the wash is that it doesn’t require swabs, which are in short supply, Persing said. Read more here and here...
Diagnostic Errors, Maternal Health Top ECRI’s 2020 Patient Safety Concern
Clinical Lab Products, March 2020 - ECRI, an independent, nonprofit organization dedicated to improving the safety, quality, and cost-effectiveness of care across all healthcare settings, recently released its report on Top 10 Patient Safety Concerns 2020. Diagnostic errors and maternal health were in the top two spots. The annual report helps organizations identify looming patient safety challenges across the continuum of care and includes suggestions and resources for addressing them. “Unsafe healthcare delivery harms millions of patients,” says Marcus Schabacker, MD, PhD, president and chief executive officer of ECRI. “Our annual patient safety report provides a roadmap to help healthcare leaders know what goes wrong and how to prevent harm.” ECRI’s Top 10 Patient Safety Concerns relies on the analysis of... Read more >
The Expanding Role of Point-of-Care Testing in Patient Care
Medical Laboratory Observer (MLO)
Over the past 50 years, we have witnessed a rapid evolution in the availability of point-of-care laboratory testing. Point-of-care (POC) testing is best defined as laboratory testing near the patient, which rapidly provides results for immediate patient care management. There are several reasons for growth in the near patient testing market: The growth of electronic medical records (EMRs) provides a central repository for all patient care information. This, in turn, has made the use of point-of-care technology more attractive in hospitals, offices, clinics, urgent care centers and emergency departments, because the information obtained at the various locations can be more widely available to multiple providers. Read more >
Errors in Point-of-Care Testing: An Alladin's Cave of Treasures
Omni Health - Traditionally, in most point-of-care testing (POCT) programmes, there has been a large focus on Quality Control (QC) analysis. Through regular monitoring of internal QC and proficiency testing reports, we can evaluate the performance of instruments. We can identify any inaccuracies and/or imprecision that may exist and take appropriate action. For the most part, instruments provided by long-established manufacturers perform very well. Reviewing QC summary data, I rarely see problems from one month to the next. If we were to focus exclusively on the QC performance of our devices, we would be fooled into thinking that all is well in the world of POCT.While the analytic performance of our instruments must...Read more >
Personal Paradox and More: POC Pitfalls
CAP Today
Point-of-care testing makes up only about 10 percent of all laboratory testing but the aggravation factor and number of people involved far exceeds that, said Deborah A. Perry, MD, medical director of pathology at Methodist Hospital in Omaha, Neb., speaking at CAP19 and calling POC testing “a whole different world.”
In a session titled “Point-of-care testing pitfalls: what you don’t know can hurt you,” Dr. Perry and Brad S. Karon, MD, PhD, professor of laboratory medicine and pathology and co-director of the point-of-care program at Mayo Clinic, used scenarios to illustrate point-of-care testing risks and how to mitigate them. “Initially, people kind of let the point-of-care side of the world go to the medical technologists, and the laboratory medical directors hoped we wouldn’t have to worry about it too much,” said Dr. Perry. Read more>
Dodging POCT Potholes in PT, IQCP
CAP Today
For point-of-care testing, perform proficiency testing on only one method or instrument unless your testing procedure says all patient samples must be tested on multiple instruments. And if a single IQCP is written for more than one POC testing location, account for all variations.These and other tips come from a CAP19 session, “Point-of-care testing pitfalls: what you don’t know can hurt you,” presented by Deborah A. Perry, MD, medical director of pathology at Methodist Hospital in Omaha, Neb., and Bradley S. Karon, MD, PhD, chair of the Division of Clinical Core Laboratory Services, Department of Laboratory Medicine and Pathology, Mayo Clinic. They used scenarios to illustrate how best to approach PT, the IQCP, and CAP inspections for POC testing. (Part one is published in the November issue of CAP Today.) Read more >
20 Years After 'To Err Is Human'
Healthcare Innovation
Organization seeing fewer deaths from preventable errors it monitors
Coinciding with the 20th anniversary of the Institute of Medicine’s groundbreaking “To Err is Human” report, the Leapfrog Group’s fall 2019 Hospital Safety Grades highlight progress on patient safety. The Leapfrog Hospital Safety Grade is a bi-annual grading assigning “A” through “F” letter grades to general acute-care hospitals in the U.S. It is the nation’s only rating focused entirely on patient safety—preventable errors, accidents, injuries and infections. Read more >
POCT Professional Certification Validates Expertise in the Field
KAREN BLUM OCTOBER 24, 2019, MLO
At Aculabs, an East Brunswick, New Jersey-based company that services long-term and acute-care facilities, laboratory director Rita Khoury, MD, DABCC, FAACC, CPP, spends her days overseeing testing for these entities, with the goal of turning around test results quickly to help reduce hospital readmission among residents. This includes a point-of-care testing (POCT) program started in 2014, which became the first and only laboratory program in the Mid-Atlantic that can help maintain, train and integrate bedside blood analysis.
So when Khoury saw an email from AACC last year announcing its new POCT Professional Certification, she was very excited, and applied immediately. Khoury became one of the first POCT professionals to take the exam last November, and soon added the CPP (certified point-of-care testing professional) initials to her name. Read more >
New test diagnoses Lyme disease within 15 mins
MLO, October 2019
Some 300,000 people in the U.S. are diagnosed with Lyme disease every year. Caused by Borrelia burgdorferi and transmitted by the bite of infected Ixodes ticks, the disease if left untreated can cause serious neurologic, cardiac, and/or rheumatologic complications.
Current testing for Lyme disease, called the standard 2-tiered approach or the STT, involves running two complex assays (ELISA and western blot) to detect antibodies against the bacterium, and requires experienced personnel in a lab, and a few hours to carry out and interpret. A team led by Sam Sia, professor of biomedical engineering at Columbia Engineering, has developed a rapid microfluidic test that can detect Lyme disease with similar performance as the STT in a much shorter time—15 minutes. Read more >
The New Wave of Diabetes Management
Monitoring Technologies Surge as Disease Prevalence Mounts
By Whitney J. Palmer, Clinical Laboratory News, October 2019
Big data and bioengineering advances are fueling rapid changes in diabetes technologies, which offer the promise of better self-management and quality of life for individuals with the disease, and easier care oversight by physicians. With the incidence of diabetes rising, these innovations are coming into use when “the ability of an individual living with diabetes to have human-to-human contact with their healthcare provider is not keeping pace with the number of people developing diabetes,” according to a recent review. Read more >
New Requirements for Molecular Micro Waived Testing
By Karen Lusky, CAP Today, September 2019
Four new checklist requirements for waived molecular-based microbiology tests have been added to the CAP point-of-care testing, limited service laboratory, and immunology accreditation program checklists, as part of the 2019 checklist edition released this month.
“The CAP has decided to improve patient care by providing additional safeguards that wouldn’t necessarily be performed otherwise,” says Bobbi Pritt, MD, MSc, DTM&H, chair of the CAP Microbiology Committee and professor, Mayo Clinic Alix School of Medicine.
Dr. Pritt and other members of the Microbiology Committee have noticed that even though the new cartridge-based waived molecular tests are self-contained, and the risk for nucleic acid leakage and contamination is low, nucleic acid contamination can occur. Read more >
CPOCT Division Announces 2019 Awards!
Kathleen David - TriCore System POCT Manager is the 2019 Point of Care Coordinator of the Year
 Kathleen is the POCT Manager at Tricore, responsible for general operation of assigned Point-of-Care Testing (POCT) at TriCore Sites: 15 hospitals and 140+ clinics. She manages the department and coordinates activities with other areas of the organization and with internal and external customers to ensure that quality standards are met. Kathleen is the POCT Manager at Tricore, responsible for general operation of assigned Point-of-Care Testing (POCT) at TriCore Sites: 15 hospitals and 140+ clinics. She manages the department and coordinates activities with other areas of the organization and with internal and external customers to ensure that quality standards are met.
She is responsible for ensuring service quality to the satisfaction of the hospital or client. She works closely with appropriate medical/scientific director(s), Site Managers and Rapid Response Lab (RRL) Director regarding decisions on matters relating to patient care, technical performance, quality and finances. She oversees and directs the efforts of POCT Coordinators to ensure goals and standards of the sponsors are met.
Rick Import - Whitehat Communications, is the 2019 recipient of the Outstanding Contributions to POCT Award!
 Rick founded Whitehat Communications in 2008 to fill an educational need for Point-of-Care Coordinators (POCCs). Rick founded Whitehat Communications in 2008 to fill an educational need for Point-of-Care Coordinators (POCCs).
Prior to Whitehat, Rick led interactive and educational discussions at POC Groups throughout the country titled "Leadership Communication" which provided attendees with techniques and tips to improve their communications with their supervisors and operators using real-life issues in the lab and on the floor.
Rick formed Whitehat Communications with one sole purpose – to produce webinars for the hospital laboratory and point of care community across the U.S. that would provide educational sessions covering a wide range of timely and practical topics chosen by the POC groups themselves.
Challenging the Status Quo on Quality Control
A focus on patient risk is driving changes to old paradigms
By Kimberly Scott, Clinical Laboratory News, June 2019
Despite knowing that errors in testing can lead to serious patient harm, too many clinical laboratories are performing only the minimum amount of quality control (QC) required by regulation and recommended by manufacturers, leading some in the industry to call for labs to adopt more robust statistical quality control (SQC) approaches designed to focus on patient risk.
A recent study of current SQC practices in U.S. laboratories found that 21 leading academic laboratories surveyed typically employ two standard deviation (SD) control limits in spite of their known high false rejection rate. It also found that labs generally use a minimum number of control measurements per run (two) and often perform the minimum frequency of SQC, explained James Westgard, PhD, founder of Westgard QC (Am J Clin Pathol 2018;150:96-104). “Based on this survey, it appears that current QC practices are based on mere compliance to CLIA minimums, rather than the best practices for patient care,” Westgard said. Read more >
A focus on patient risk is driving changes to old paradigms
By Kimberly Scott, Clinical Laboratory News, June 2019
Despite knowing that errors in testing can lead to serious patient harm, too many clinical laboratories are performing only the minimum amount of quality control (QC) required by regulation and recommended by manufacturers, leading some in the industry to call for labs to adopt more robust statistical quality control (SQC) approaches designed to focus on patient risk.
A recent study of current SQC practices in U.S. laboratories found that 21 leading academic laboratories surveyed typically employ two standard deviation (SD) control limits in spite of their known high false rejection rate. It also found that labs generally use a minimum number of control measurements per run (two) and often perform the minimum frequency of SQC, explained James Westgard, PhD, founder of Westgard QC (Am J Clin Pathol 2018;150:96-104). “Based on this survey, it appears that current QC practices are based on mere compliance to CLIA minimums, rather than the best practices for patient care,” Westgard said. Read more >
The Evolution of Leadership in POCT
By Kimberly Scott, Clinical Laboratory News, July/August 2019
As point-of-care testing (POCT) continues to grow in hospitals, physician offices, retail clinics, and other settings, professional roles are also evolving to keep pace with changes in this technology, the complexity of testing, and the need to engage with clinicians.
Once consisting of just a handful of assays, POC tests now number in the hundreds, ranging from blood glucose monitoring to rapid strep to prothrombin time/international normalized ratio (PT/INR). The market for POCT grew by an estimated 9.3% between 2013 and 2018, and worldwide, the POCT and rapid diagnostics market is projected to top $38 billion by 2022, according to industry experts.
With the growth of POCT comes challenges for laboratory directors, POC coordinators, and other professionals responsible for ensuring that instruments are maintained and that those performing testing are trained, follow quality control protocols, and remain in compliance with clinical and regulatory standards.
Brenda Suh-Lailam, PhD, DABCC, FAACC, director of clinical chemistry and point-of-care testing at the Ann & Robert H. Lurie Children’s Hospital of Chicago, said much of her time is spent ensuring the quality of testing done at patients’ bedsides by defining, implementing, and monitoring the standards of performance. Read more >
Mass Casualty Plan Puts POCT in the ED
Jayne O'Donnell, USA TODAY May 15, 2019
If a mass casualty event brings patients to Le Bonheur Children’s Hospital in Memphis, Tenn., clinical laboratory staff will head straight to the bedside. Le Bonheur Children’s Hospital is a level-one trauma center. Its new mass casualty response plan, two years in the making, has laboratory staff in the emergency department and triage areas, where they will perform point-of-care testing for frontline providers.
“Having medical lab scientists just sitting in the lab waiting for blood to come to them made no sense,” says laboratory director Lisa M. Griffin, BS, MT(ASCP). “Instead of keeping them away from where all the injured patients are, we decided to send the techs to them. It’s the best way to use the trained, professional human resources we already have.” Read more>
Low rated US Hospitals are deadlier due to mistakes, botched surgery, infections
Jayne O'Donnell, USA TODAY May 15, 2019
The Leapfrog Group's latest hospital safety rankings gave 168 US hospitals out of about 2,600 failing or near-failing grades, while about one-third got A grades. A report from the Armstrong Institute for Patient Safety and Quality at Johns Hopkins Medicine, based on the Leapfrog data, found about 160,000 people die a year from avoidable medical errors, down from 205,000 in 2016. Read more >
Glucose Meters: Current Regulatory Guidance for Manufacturers and Providers
April 2019, MLO, By Jeffrey A. DuBois
Therapeutic management of blood glucose in patients with diabetes in the home or in the hospital involves the use of glucose meters for the rapid assessment of whole blood glucose. On Oct. 11, 2016, the U.S. Food and Drug Administration (FDA) published guidance documents for glucose meters. These guidance documents are for manufacturers, not for providers. The Centers for Medicare and Medicaid Services (CMS) and its designees provide accreditation (certification) for and oversight of provider compliance under the Clinical Laboratory Improvement Amendments (CLIA) of 1988. This is an important distinction for all stakeholders involved in the management of dysglycemia in hospitalized patients, many of who are on intensive insulin therapy to achieve safe and effective glycemic control.
The FDA guidance documents make a clear distinction between devices that are designed, cleared, and classified for self-monitoring of blood glucose in the home and devices that are designed, cleared, and classified for blood glucose monitoring for prescription point-of-care (POC) use. It took six years for the FDA to publish these guidance documents for manufacturers. Read more >
MLO Selects 2019 Lab of the Year!
Penn Medicine Lancaster General Health
Selecting a Lab of the Year is never an easy task thanks to the outstanding nominations MLO receives year after year. And since history tends to repeat itself, 2019 was no different. With much respect and admiration, MLO is proud to present the 2019 LOY winner: Penn Medicine Lancaster General Health Laboratory. Located in Southern Pennsylvania, approximately 70 miles west of Philadelphia, sits Lancaster General Health Laboratory, part of Lancaster General Hospital (LGH). This 533-bed, nonprofit hospital is part of Lancaster General Health/Penn Medicine, a member of the University of Pennsylvania Health System (Penn Medicine). Read more >
Critical Points - AACC Critical and POCT News
The latest edition of the CPOCT Division Newsletter has been posted to the CPOCT Division webpage. Please navigate to the Division webpage within AACC.org, or copy and paste this link below to take you directly there...www.aacc.org/community/divisions/... Highlights include: Division Awards Nominations and upcoming 2019 CPOCT division activities not to be missed!
POC Roundtable: Risks, Resources, Relationships
CAP Today, March 2019
Infection control and the heavy demands on point-of-care coordinators were among the top concerns that came up in a recent CAP TODAY roundtable on point-of-care glucose testing. CAP Publisher Bob McGonnagle spoke with four POC testing experts: Sharon Geaghan, MD, Cynthia Bowman, MD, Steven Cotten, PhD, and Corinne Fantz, PhD. Read what they had to say..
HealthGrade's Best Hospitals for 2019
By HealthLeaders Media, February 2019
Healthgrades released its annual list Tuesday of "America's Best Hospitals," using clinical outcomes to identify the top 5% of hospitals nationwide.In years past, Healthgrades released two separate awards, one for the top 250 hospitals in clinical excellence and the other for the the top 50 and 100 hospitals. But this year is the first time the two awards have been consolidated into a single list.
"Consumers have many options for care, so when hospitals prove their long-term commitment to the patient and to achieving high-quality clinical outcomes, it sets their system apart in a sea of choices," Healthgrades Chief Medical Officer Brad Bowman said.
To see the top 50 hospitals for 2019, as identified by Healthgrades and grouped by state,
click here >
Complimentary Training Support for Urinalysis and Diabetes
Siemens Healthineers is pleased to announce the availability of complimentary training support tools for our point-of-care chronic disease management portfolio. Listed below are the resources available to you. These online options provide you with easy access to in-service product training, forms, and procedures for your staff.
For the CLINITEK Advantus® Urine Chemistry Analyzer, CLINITEK Status® family of analyzers, DCA Vantage® Analyzer and Xprecia Stride™ Coagulation Analyzer, we offer the following:
Sign up for Remote Video Training: Receive telephone or video training from a medical technologist. To schedule your complimentary training session, email or call 1 (866) 748-7463 to speak with a training specialist.
Individualized, Competency-based Education: Click here to register for personalized product training modules and assessment tools.
In-service Training tools: Click here to conduct in-house training events for your staff and access product-specific training and support documents to maintain your audit-readiness.
Navigating the Quandaries of Coagulation Testing
CAP TODAY January 2019, by Anne Paxton
Naming the things about coagulation testing that most perplex clinicians isn’t easy for Michael Laposata, MD, PhD. But there’s a good reason for that: He finds confusion to be pervasive. New drugs with untoward effects on traditional coagulation tests, revamped clinical guidelines, and assays that can be difficult to interpret have been among the more recent contributors to clinicians’ bewilderment. Dr. Laposata, however, sees a more basic problem: “All of coagulation testing is confusing for the average physician in all specialties.”
It’s been that way for some time, says Dr. Laposata, chairman of the pathology department at the University of Texas Medical Branch at Galveston—and he should know. He’s been a faculty member at different institutions since 1985, but even earlier, on his first encounter with clinicians as a resident, he saw there was a knowledge gap. “I realized that the doctors on the floor didn’t know how to interpret even the simplest coag test result—even the brightest doctors. To me, that was a shock.” He found they needed to know not only which test to order but also what the results meant and what the recommended next steps would be. Read more >
Hemoglobin A1c Testing and Diabetes Management
By: Jessica Pawlak, Michael Sweatt, Catherine Cahill, Ralph Ito, December 21, 2018, MLO
The Diabetes Research Institute Foundation has estimated a 50 percent increase in the number of people living with diabetes mellitus in the United States over the past decade. With more than 400 million people living with and managing diabetes worldwide, the ability to accurately diagnose and track patient management is a growing need. The diagnosis of diabetes mellitus uses a combination of measurements: fasting serum glucose levels, presentation of symptoms, two-hour plasma glucose levels during a glucose tolerance test, and hemoglobin A1c (HbA1c) levels.2 Current patient management includes diet, exercise, medication, daily monitoring of blood glucose, and HbA1c monitoring.
Mayo Clinic Laboratories emphasizes the value of controlling glucose levels to prevent long-term complications such as retinopathy, neuropathy, and cardiovascular disease. However, solely measuring and monitoring blood glucose levels has some limitations as the test only measures glucose levels at the time of testing and it relies on the patient to consistently test their levels at home, using a point-of-care device. To address these limitations and provide a broader indication of long-term glycemic control, HbA1c testing is used. It is typically performed in a laboratory setting and the test indicates the patient’s average levels of blood glucose over the past 8 to 12 weeks. The NGSP, originally called the National Glycohemoglobin Standardization Program, supports the American Diabetes Association’s recommendations that patients who are meeting glycemic goals be tested for HbA1c twice a year, while patients not meeting glycemic goals or patients with changes to therapies be tested every three months. The American Diabetes Association sets a normal patient at < 5.7 percent, prediabetes patients at ≥ 5.7-6.5 percent, and diabetic patients at ≥ 6.5 percent HbA1c. Read more >
Rapid PCR Rules as Labs ready Flu Arsenal
December 2018, CAP Today, Anne Paxton
With the memory of the 2017–2018 “high-severity” influenza season fresh in mind—49 million cases, 960,000 hospitalizations, a marginally effective vaccine, 79,000 deaths—clinical laboratories have been bracing for the customary annual surge in emergency room, outpatient clinic, and physician office influenza test orders. Although flu admissions have been rising somewhat, it is too soon to know how the season will play out, but laboratories are hoping for a season closer to average.
Avoiding a repeat of last year’s travails—lengthy turnaround times, supply shortages, and the need to triage patients for testing—is a must, many laboratory directors say. “We had difficulty keeping up with last year’s demand. It was extremely time-consuming,” says Mary Kay O’Connor, national laboratory director at Summit Health Management, the management arm of the Summit Medical Group, an 800-provider practice on the East Coast. Read more >
The Power of Internal Audits and Site Inspections
for Improving Point-of-Care Testing
By
Vikram Palamalai, PhD, November 2018, Clinical
Laboratory News
At MetroHealth, a county-wide healthcare system
based in Cuyahoga County, Ohio, the volume and
complexity of our point-of-care testing (POCT) have
increased rapidly. In the past, we had a
decentralized POCT system in which individual
testing centers held independent CLIA licenses.
While this was a reasonable strategy when POCT was
available only at a few sites, the mushrooming
dissemination of this modality led to a situation in
which our POCT coordinators could provide only
initial training and guidance with technical issues
but were not involved in overseeing individual
testing areas.
Though qualified medical staff were designated as
directors, oversight was lacking at some sites. The
limitations of this decentralized system came into
sharp focus during an accreditation visit that found
significant shortcomings, primarily due to the
fragmented nature of the POCT program. Read more >Improve Point-of-Care Glucose Measurements with
Staff Outreach
By Uyen
B Chu, PhD and Tiffany N Heady, PhD, Medical Lab
Management, October 2018
Bedside glucose testing using strip technologies
constitutes the highest test volume in most
hospital-based point-of-care testing (POCT)
programs. A 2010 study estimated that in health care
settings, 51 percent of POCT involves bedside
glucose meters using strip technologies. However,
since the 2014 published warnings from the US FDA
and CMS (both since retracted) on the potential
erroneous results produced by glucose meters in
critically ill patient populations, managing the
off-label use of bedside glucose testing presents a
significant challenge for hospital POCT programs.
Issues Exacerbated in Critically Ill
Originally designed as an over-the-counter product
for managing glucose levels in diabetic patients,
glucose meters are now used on virtually every
hospital patient regardless of medical condition or
the limitations specified in the manufacturer’s
package insert. Glucose meters were initially... Read more >
The Pros and Cons of Point-of-Care Testing versus Laboratory Testing
By
Lisa-Jean Clifford, MLO
Point-of-care
testing (POCT) is used to refer to any patient
testing that is done at, or near, the actual
location of the patient. But how does
software enable these testing capabilities, and are
the results comparably applicable to the results in
the lab?
Read more >
The Laboratory’s Role in Guiding the Best Use of
Point-of-Care Testing
By Kim
Futrell, MLO
Continued growth in point-of-care testing (POCT) is
certain as technologies improve and the benefits of
POCT are realized in value-based healthcare.
However, POCT is a diverse and complex area of
laboratory testing, riddled with the challenges
inherent to multiple locations, disparate devices,
and non-laboratory trained operators. To reap the
advantages POCT has to offer, POCT programs can
greatly benefit from laboratory intervention and
oversight. Laboratory professionals who approach
POCT oversight as a team endeavor, keeping end
users’ workflows and backgrounds in mind, can be
instrumental in helping reap the potential benefits
that POCT offers in patient care.
POCT’s renewed value instigates continued growth
The rapid turnaround time (TAT), convenience, and
mobility of POCT—in specific patient scenarios—can
speed clinical decision-making and treatment
decisions and simultaneously help avoid other
unnecessary procedures and associated risks. POCT’s
rapid results can help optimize which patients
receive advanced care, and improve patient
understanding and engagement, giving POCT a more
important role in patient-centered care. Read more >
Create a Strong Lab Team Through Recruiting
By
Milly Keeler, MT(ASCP), CLC(AMT), CCCP, Medical
Laboratory Management
No
matter how much money is spent on sophisticated
laboratory instrumentation, a lack of qualified,
well-trained personnel will undermine the
laboratory’s success at every turn. In fact,
well-trained and skilled laboratory personnel are
the single greatest determining factor of
operational success. That said, recruiting and
retaining new laboratorians can be difficult, time
consuming, and expensive.
Managing risk is more important than ever in this
litigious age and as experienced laboratory staff
members are retiring faster than new employees are
able to fill those positions, many laboratories are
experiencing significant increases in workload and
work-related stress. These circumstances are a
breeding ground for potential mistakes, increased
costs due to overtime and temporary workers, and for
the cessation or abandonment of improvement
projects. Therefore, a concerted effort should be
invested in how the laboratory is bringing new staff
on board. Read more >
Top 10 Health
Technology
Hazards for 2019
Health Data
Management,
October 2018
Health
technology—ranging
from simple
devices to
complex
information
systems—poses
unanticipated
risks for
healthcare
organizations.
It’s
important to
identify
these risks,
understand
them and try
and correct
them. Each year,
the ECRI
Institute’s
Health
Devices
Group
produces a
list of the
top 10
health
technology
hazards,
identifying
potential
sources of
danger that
warrant the
most
attention
for the
coming year.
“All the
items on our
list
represent
problems
that can be
avoided or
risks that
can be
minimized
through the
careful
management
of
technologies,”
ECRI
reports. Read more
>
The Evolution of Group A Streptococcus
Pharyngitis Testing
By
Dithi Banerjee, PhD, and Rangaraj Selvarangan, BVSc,
PhD, D(ABMM), FIDSA Sept 2018, Clinical Laboratory
News
Molecular assays may
soon eliminate the need for supplemental testing,
but patient selection and appropriate test methods
remain key
Acute pharyngitis,
an inflammation of the pharynx and/or tonsils, is a
common illness caused by many microorganisms.
Although viruses are the main etiological agents,
Streptococcus pyogenes, commonly known as group A
streptococcus (GAS), is the primary bacterial cause,
accounting for pharyngitis in 5%–15% of adults and
20%–30% of children worldwide (1). GAS pharyngitis mainly affects children 3–15 years
of age and can lead to suppurative and non-suppurative
complications, the latter being more common in
developing countries. Suppurative complications
include oral or peritonsillar abscesses, cervical
lymphadenitis, and rarely, septicemia. Read more >
POC in the Lab: A Regional Experience in
Urinalysis and Pregnancy Testing
MLO, Yu
Chen, Susan McDonald, and Jason Weshler
Horizon
Health Network operates 12 hospitals and more than
100 medical facilities, clinics and offices in the
province of New Brunswick in Canada. Providing
services ranging from acute care to community-based
health services, Horizon Health Network has more
than 12,400 employees, including 1,000 physicians,
and has 5,700 volunteers, auxiliary workers, and
alumni. The network consists of four areas with core
lab services provided only in the four regional
hubs. As part of the provincial laboratory
standards, all sites performing point-of-care
testing (POCT) must be accredited by the Institute
for Quality Management in Healthcare IQMH (former
Ontario Laboratory Accreditation 2013). Findings
from a 2013 audit identified minor non-conformances
specific to ISO 22870 in urinalysis POCT, which
remained unresolved during a subsequent audit in
2015 and were escalated to major non-conformances. Read more > A Novel Point-of-Care Approach for
Improving Acute Bleeding Management
MLO, By Todd Allen and Francesco Viola
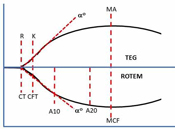 Whole
blood viscoelastic testing (VET) for
perioperative bleeding management is
systematically increasing in clinical
use and is approaching the level of
standard of care for many clinical
settings such as cardiovascular surgery,
liver transplantation, trauma, and
obstetric hemorrhage. Conventional
coagulation testing has proven to be
inadequate for directing therapeutic
intervention in these critical settings. Whole
blood viscoelastic testing (VET) for
perioperative bleeding management is
systematically increasing in clinical
use and is approaching the level of
standard of care for many clinical
settings such as cardiovascular surgery,
liver transplantation, trauma, and
obstetric hemorrhage. Conventional
coagulation testing has proven to be
inadequate for directing therapeutic
intervention in these critical settings.
Physicians managing acute bleeding
events require faster turnaround times
for test results and prefer assays that
more accurately reflect the whole blood
or cell-based hemostatic process
described by Hoffman and Monroe. The
benefits of VET have been
well-documented. There exists an
abundance of publications and systematic
reviews in a variety of clinical
settings, including review articles in
this area published in previous issues
of MLO. Several medical societies have
given strong recommendations for the use
of VET in conjunction with goal-directed
treatment algorithms guided by VET for
managing acute bleeding in the
perioperative setting. To date, two
technologies have emerged at the
forefront of whole blood VET:
thromboelastography and rotational
thromboelastometry. Read more >
One and Done?
Prospective
trial suggests that a single blood test may be
sufficient to diagnose diabetes.
Clinical
Laboratory News, July 2018
Multiple blood tests have been the clinical mainstay
for confirming type 2 diabetes. However, a study
that tracked individuals over several decades for
incident diabetes and other conditions found that
measuring elevated fasting glucose and HbA1c levels
from a single blood sample may suffice for an
accurate diagnosis. Investigators published the
results of their prospective cohort study in the
Annals of Internal Medicine.
Clinicians under current guidelines rely on two
glucose tests to confirm a diabetes diagnosis.
“Whether 2 different tests from a single blood
sample provide adequate confirmation is uncertain,”
wrote the study’s investigators, who launched a
prospective study known as the Atherosclerosis Risk
in Communities (ARIC) trial to see if this approach
was possible.
Read more...
The FDA reviews guidelines for capillary glucose
testing in critically ill patients
By
Jeffrey A. DuBois, MLO, June 21, 2018
Capillary whole
blood testing with point-of-care (POC) glucose
meters in hospitalized patients and, particularly,
in critically ill patients, remains a topic of
interest in the medical and regulatory communities.
However, determining the requirements for effective
clinical use has proved challenging.
An FDA panel
convenes
This past March,
the U.S. Food and Drug Administration (FDA) convened
its Clinical Chemistry and Clinical Toxicology
Devices Advisory Panel, seeking guidance and
recommendations on the acceptability of capillary
specimens in critically ill patients based on
benefits and risks, and whether capillary specimen
testing in this patient population meets the
criteria for waived status under the Clinical
Laboratory Improvements Amendments (CLIA)
regulations. The FDA began by summarizing the
history of POC glucose testing for the panel and
emphasized the need for manufacturers to submit data
supporting their glucose meters’ acceptability for
use with critically ill patients. The FDA reviewed
the data submitted for a glucose meter cleared for
use with these patients using arterial and venous
specimens, and related that no manufacturer had
submitted data for capillary whole blood. Read more >
The clinical laboratory
is an inherently
dangerous place.
Laboratorians face a
variety of dangers
working in an
environment that
contains biohazards.
Utilizing standard
precautions and
correctly employing
Personal Protective
Equipment (PPE) are
essential keys to ensure
laboratorians’ safety.
Maintaining a clean and
orderly environment and
employing good
disinfection practices
are vital as well. A
cluttered workspace and
an area contaminated
with biohazards threaten
the safety of both
employees and visitors.
General disinfection
tips
Lab directors should
conduct audits of their
department’s physical
environment to identify
safety hazards specific
to their lab. Such
audits typically do not
need to interfere with
the day-to-day lab
processes, and they
should be performed on a
regular basis, at least
monthly. Many changes
can occur in a
laboratory at any time,
such as the movement of
instruments, the
placement of new
equipment, or even the
movement and stocking of
lab supplies, and the
implications of such
changes for safety
should be recognized. Read more >
New guidelines and studies suggest improved
approaches to C. difficile testing
By
Sherry A. Dunbar, MLO, June 21, 2018
Clostridium
difficile represents a significant health threat
around the world. In the United States, infections
caused by C. difficile are now the most common type
of healthcare-associated infection. Nearly half a
million infections occur in the U.S. annually, with
an estimated 29,000 deaths within 30 days of the
initial diagnosis.
Consequently, much effort is ongoing toward the
development of better testing and treatments for C.
difficile. This year, new clinical guidelines were
released that included significant changes to how
healthcare teams respond to C. difficile infections.
In addition, scientists and clinicians are
conducting a number of studies and generating useful
information that could guide new expectations or
policies about testing and treatment.
For example, studies have shown that molecular tests
targeting a marker specific to a single C. difficile
strain are less useful now, as other strains of the
pathogen have become more prevalent.3-5 These
findings could help clinical labs fine-tune their C.
difficile testing procedures to ensure the most
reliable results. Also, several recent studies have
demonstrated that... Read more >
Christiane Nooney from Duke Hospital
Named 2018 POCC of the Year by AACC
The
AACC Critical and Point of Care Testing (CPOCT)
Division has announced that Christiane “Chris”
Nooney, MBA/MHA, MT(AMT), DUH POC Supervisor, Duke
Hospital, DukeHealth has been award the 2018 Point
of care Coordinator of the Year.
Chris was unanimously selected as the 19th recipient
of this auspicious award given annually to recognize
outstanding achievements in the POCT field by
persons who are primarily responsible for a given
institution’s POCT program. It is based on the
extent of the nominee’s responsibilities and
accomplishments, particularly the impact this person
has made in improving the quality of the POCT
program at their facility. The award also includes a
cash award and funds to support attendance at the
AACC Annual Meeting as well as an elegant trophy. Read more >
Point of Care Testing Compliance
How Partnering
With Nursing Leadership and Sharing Data Upped
Performance on a Crucial Parameter
By Adil
I. Khan, MSc, PhD, Clinical Laboratory News, June
2018
The of the hardest aspects of point-of-care testing
(POCT) is trying to make the diverse users of POCT
devices follow written procedures and perform
testing exactly as stated by manufacturers. The
simplicity of POCT devices, often involving
disposable kits with no maintenance or
troubleshooting, tempts users to take shortcuts. The
downside of this approach is that when procedures
are not followed to the letter, mistakes happen.
POCT devices are designed so they can be used by
anyone with at least a high school diploma, hence
users range from students to physicians. Read more >
Preanalytical Errors and Critical Variables in
Point-of-Care Testing
By
Aparna Jha Ahuja, MD, May 24, 2018, MLO
Today’s “smart” technology enables us to have
important information at our fingertips.
Point-of-care testing (POCT)—also referred to as
“near patient, bedside, and extra-laboratory
testing”1—offers the rapid delivery of healthcare
information as well.
Centralized laboratory testing was the standard
until the mid-1980s. Since that time, many
laboratory tests (e.g., glucose and blood gas
testing) have transitioned to patient care settings,
including physicians’ offices, ambulances, and
hospital units (e.g., the intensive care unit,
emergency department, surgical suites), as well as
clinics, dialysis centers, and nursing homes.2
Devices for POCT range in size from small handheld
meters for glucose monitoring to larger benchtop
analyzers for hematology. Read more >
Field-Portable MDx
MLO, By
John Brunstein, May 24, 2018
There is an
enduring appeal to the concept of point-of-care (POC)
or near-POC diagnostic methods. Having the ability
to perform a diagnostic test in the doctor’s office
while a patient is present, rather than having to
send a sample off to a centralized lab for testing,
means that what would otherwise need to be two
patient visits could be replaced by a single
session. It also suggests the potential for a more
timely response with a specific rather than
empirical treatment strategy, with particular
implications for the appropriate, limited use of
antibiotics. Carrying the POC concept a step
further, one can imagine the potential utility if
cheap, effective, reliable diagnostic systems could
be made small, portable, simple, and rugged enough
for use in low-resource settings, where they might
have the greatest human impact.
Of course, many
such diagnostic methods exist, but they are most
frequently some form of a rapid immunological test.
While these excel in simplicity, low cost, and
speed, they generally lack the sensitivity and
specificity that a molecular method would provide.
That they are so widely used even with these
shortcomings underscores the need for POC/near-POC
testing and the potential for growth in this field
if suitable molecular devices and tests can be
developed. Read more >
CMS gives 213 hospitals 'five
stars' for patient experience. See how yours fared...
May
2018 | https://www.advisory.com/daily-briefing/2018/05/08/hcahps-star-ratings
CMS on April 25 updated its Hospital Compare website
with new Hospital Consumer Assessment of Healthcare
Providers and Systems (HCAHPS) summary star ratings.
CMS' summary star rating scores hospitals on a
one-to-five-star scale based on the 11 publicly
reported measures in HCAHPS survey, which assesses
patient experiences. The agency started assigning
hospitals patient experience star ratings based
solely on HCAHPS scores in April 2015. The latest
update is based on HCAHPS survey data collected
between July 1, 2016, and June 30, 2017.
4 ways patient experience may be costing you
The patient experience summary star ratings are
distinct from CMS' overall quality star ratings,
which are scheduled to be updated in July. Overall
star ratings are based on 62 quality measures from
seven categories: effectiveness of care, efficient
use of imaging, mortality, patient experience,
readmissions, safety, and timeliness of care.
A map of the country’s hospitals and
their rankings is available on the Advisory Board’s
Web site (registration is required to view the map). Read more >
Considerations for Implementing New POC Testing
Tyler
Gledhill, BS, Robert L. Schmidt, MD, PhD, MBA,
Brenda VanCleve, MT(ASCP), Sandra K. White, MS,
Medical Laboratory Management, Clinical Leadership &
Management Review
Point-of-care testing (POCT) can deliver significant
benefits to both patients and providers, and due to
this, POCT has experienced rapid growth in recent
years. While the end result of POCT can be quite
positive, proper implementation and management can
present challenges and requires vigilant oversight
to ensure success. Regardless of whether the
organization is new to POCT or has a fully
functioning POCT department, implementing a new POC
test requires careful planning. Test implementation
can raise unique issues that may be unfamiliar to
laboratory and hospital staff. These include
consideration of federal and state regulations,
relationships with regulatory and accreditation
bodies, POC test management and technical
performance, and overall fit with the organization.
Taking an administrative viewpoint, laboratory
directors must focus on test justification and
dispersion when considering a new POCT. Before
approving an application to implement POCT, it is
key that laboratory leadership consider the
following issues.
Determine
Necessity and Benefit
Requests for new POC tests typically originate from
clinicians who desire more expedited results.
Ideally, a rapid result enables physicians to
provide a diagnosis or prescribe a treatment at the
time of the patient encounter. This can reduce the
time to therapy, increase adherence, and reduce the
potential for errors in handling specimens. However,
managers should exercise caution before instituting
new POCT, as the benefits are highly dependent on
the context in which the test will be implemented. Read more >
American College of Physicians
Recommends Less Restrictive HbA1c
Targets
The Sample: May 2018, Clinical
Laboratory News
In a controversial new clinical
guideline, the American College of
Physicians (ACP) recommends less
restrictive HbA1c targets for
glycemic control in most patients
with type 2 diabetes; between 7% and
8% rather than 6.5% or 7% as
recommended by other groups (Ann
Intern Med 2018;
doi:10.7326/M17-0939).
ACP based this advice on evidence
about the benefits and harms of
lower HbA1c targets from clinical
trials considered by the other
groups in setting their HbA1c
targets. “ACP’s analysis of the
evidence behind existing guidelines
found that treatment with drugs to
targets of seven percent or less
compared to targets of about eight
percent did not reduce deaths or
microvascular complications such as
heart attack or stroke but did
result in substantial harms,” said
Jack Ende, MD, president of ACP. Read more >
POC Glucose: Views on Volume,
Critical Care, ACOs
CAP Today, April
2018
Test volume,
limitations on devices used in critical care,
consolidation, and population health is what CAP
TODAY asked about when it spoke in March with the
makers of three bedside glucose testing systems. “Customers are
more aware than ever of the limitations that are in
the package inserts from the glucose manufacturers,”
says Corrine Fantz, PhD, director of medical and
scientific affairs for point-of-care testing, Roche
Diagnostics. But she and Kevin Peacock, clinical
marketing manager, HemoCue America, say there is
still confusion. This article
features responses to the following questions posed
by CAP Today senior editor Amy Carpenter Aquino.
-
How has the
decline in reimbursement coupled with a retreat
from tight glycemic control affected test volume
for patients at the bedside?
-
How are your
customers adapting to the limitations on glucose
devices for critical care applications?
-
How has
system consolidation—including established
system clinics, ERs, and acquired physician
practices—affected POC glucose testing for
ambulatory patient testing?
-
How does
glucose testing and the management of patients
with diabetes fit into the concern laboratories
have now for population health and accountable
care organizations?
For the complete article, click here.
| Glucose systems are profiled here.
Thinking Beyond The Instrument
Laboratories are using IQCP to bring
preanalytic and postanalytic factors
into focus and improve patient care
By Julie Kirkwood, APR.1.2018, Clinical Laboratory
News
Nearly 5 years ago, the U.S. Centers
for Medicaid and Medicare Services
(CMS) introduced a new option for
laboratory quality control (QC)
called the Individualized Quality
Control Plan (IQCP). Laboratories
could create an IQCP as an
alternative to performing two levels
of external QC per day of patient
testing (default QC), as long as
their risk assessment supported a
longer QC interval and it complied
with manufacturers’ instructions.
The catch? Equivalent quality
control (EQC), an option that had
been available since 2004, was being
eliminated. EQC allowed labs to run
external QC on a weekly or monthly
basis for tests with built-in QC
features, as long as the schedule
met minimum manufacturers’
recommendations.
Under the new rules, which took
effect in January 2016 after a
2-year educational period, labs
needed to write an IQCP for every
test that had been operating with
EQC or perform default QC.
Laboratory managers, particularly
those in charge of point-of-care
testing (POCT, were faced with the
daunting task of conducting risk
assessments and writing IQCPs for
dozens or even hundreds of tests. Read more >
IBM Watson Health Announces 100 Top Hospitals
Formerly the Truven Health Analytics 100 Top
Hospitals, 2018 Study Finds Top U.S. Hospitals
Improve Outcomes at Lower Cost and Higher Profit
Margins than Peers
IBM Watson Health™ today published its 100 Top
Hospitals® annual study identifying top–performing
hospitals in the U.S. based on overall
organizational performance. Formerly known as the
Truven Health Analytics® 100 Top Hospitals, this
study spotlights the best–performing hospitals in
the U.S. based on a balanced scorecard of publicly
available clinical, operational, and patient
satisfaction metrics and data. It has been conducted
annually since 1993.
Overall, the Watson Health 100 Top Hospitals® study
found that the top-performing hospitals in the
country achieved better risk-adjusted outcomes while
maintaining both a lower average cost per
beneficiary and higher profit margin than
non-winning peer group hospitals. Did your hospital make the list?
What's New in Point-of-Care Testing?
Point of Care: March 2018 - Volume 17 - Issue 1
By
Kantartjis, Michalis BS; Melanson, Stacy E.F. MD,
PhD
Point-of-care
testing (POCT) is one of the fastest growing sectors
in diagnostics, becoming a well-established
laboratory tool in healthcare systems across the
world. Recent research has focused on increasing
cost-effectiveness and improving overall performance
of existing POCT. Lower costs as well as rapid and
accurate results have allowed for POCT to be used in
resource-limited regions and positively impact
patient care globally. Literature on
POCT published between January 1, 2016 and December
31, 2016 was reviewed. Select articles are
summarized and grouped into the following
categories:
-
hematology
and coagulation
-
cardiac
disease
-
infectious
disease and human immunodeficiency virus
-
gastrointestinal disease
-
emergency
medicine
-
glucose and
diabetes
-
technological
advancements, and other.
Read more > Note, you have to
subscribe to the POC Journal or purchase this
article.
Labs Take Stock of Surprising Flu Season
CAP
Today, March 2018, by Amy Carpenter Aquino
In
a severe flu season that started early, laboratories
faced unprecedented test volumes, used new testing
platforms, and negotiated vendor supply shortages.
When laboratory staff at Arkansas Children’s
Hospital in Little Rock began seeing a rising number
of requests for respiratory tests, and five positive
flu results, in September 2017, they suspected they
were in for a record flu season, says Sherry
Childress, BSMT(ASCP), technical chief, molecular
diagnostics and immunology. Read more >
CDC Warns of a Second Wave of Flu Virus
By
LABline, March 28, 2018
The
flu season may be winding down, but parents of young
children have reason to remain watchful. As flu
activity continued to decrease across the nation,
the A-strain H3N2 influenza virus, which had
dominated previously, was reported less frequently
than B viruses, the CDC weekly surveillance report
indicated Friday. During the week ending March 17,
nearly 58% of all laboratory-confirmed cases of flu
were caused by B-strain viruses, according to the
CDC report. Circulating strains this season, which
began in October, were a mix of A viruses (H3N2 and
H1N1) and B viruses. Generally, the H3N2 strain
leads to more severe illness and more
hospitalizations than B strains, according to the
CDC. Read more >
MLO's 2018 Lab of the Year: St. Luke’s Health
System’s Core Laboratory
By
MLO Staff, March 2018
The
competition was tough, the judging was not easy—but
MLO is proud to present the 2018 Lab of the Year:
St. Luke’s Health System’s Core Laboratory.
St.
Luke’s Health System (SLHS) is a not-for-profit,
locally owned health system serving southern Idaho
and eastern Oregon. St. Luke’s Core Laboratory was
established in 2011 and is a department of SLHS. The
laboratory includes five physical locations in
Boise, Idaho; one main testing site, and four
outreach phlebotomy sites. Read more >
MLO’s 2018 Annual
Salary Survey of Laboratory
Professionals
How Much does a
Point-of-Care Coordinator Earn?
By: MLO Staff,
February 2018
The United States
Department of Labor, Bureau of Labor Statistics (BLS)
states that employment in “Healthcare Occupations”
is projected to grow 18 percent from 2016 to 2026,
much faster than the average for all occupations.
Healthcare occupations will add about 2.4 million
new jobs to the workforce. In fact, healthcare is
projected to add more jobs than any of the other
occupational groups. This projected growth is mainly
due to an aging population which leads to greater
demand for healthcare services. Read more >
Diabetes
Roundup
By MLO
Staff, February 22, 2018
Diabetes is not so much being cured as it is being
surrounded. Researchers are coming at this common
disorder from a number of different perspectives,
and some of their discoveries are finding their way,
or soon might find their way, into clinical
practice. MLO provides a
roundup of some recent scientific approaches
regarding type 1, type 2, and gestational diabetes
mellitus (GDM), as well as an exciting recent FDA
approval of a needle-free glucose testing device
that may make life easier for people with diabetes. Read more >
CLP Tech Guide: Lab
and POC Glucose Monitors
February 8, 2018
The Clinical Lab Products Tech Guide features lab
and point-of-care glucose monitors from such
companies as Arkray USA, Nova Biomedical, and Oak
Tree Health. The guide is available as a free download.
Flu Map Shows How
the Biggest Influenza Outbreak in Years Spread
Across the U.S.
TIME Health, By JAMIE DUCHARME and
DAVID JOHNSON January 19, 2018
With months left to go in the 2018 flu season, the
U.S. has already hit an unfortunate benchmark, as
shown on the flu map below: For the first time in
the Centers for Disease Control and Prevention’s 13
years of influenza monitoring, every state in the
continental U.S. is seeing “widespread” virus
activity.
The U.S. is experiencing such an active flu season
that the CDC held a special briefing on the topic
last week, explaining that there’s an uptick in both
confirmed cases of the disease and hospitalizations
related to it this year. The flu is so widespread,
in fact, that the agency has declared it an
epidemic, and urged those who have not been
vaccinated to seek out the flu shot. But how did
this year’s flu season get so bad? Read more >
Blood Glucose Test Strips
Another Shared
Diabetic Supply Harboring Bacterial Contamination
Clinical Laboratory News, By Sharon Geaghan, MD,
January 2018
When you or a family member are admitted to the
hospital, you expect that the room will be cleaned
and disinfected thoroughly. You do not expect to
find half-used tissue paper boxes or leftover
bandages from the previous patient. To the contrary,
patients expect that hospitals will take all
necessary precautions to avoid spreading disease,
including disposing of patients’ medications when
they are discharged from a facility.
Perhaps the only exception to the current practice
of single-use, single-patient hospital supplies is
blood glucose test strips. Hospitals and other
institutions often procure blood glucose test strips
in 25- or 50-count vials and bring them from patient
to patient and room to room for testing purposes.
Testing sites range from acute care hospitals,
outpatient clinics, skilled nursing facilities and
long term care facilities to prisons, shelters,
surgery centers, schools, and camps. Read more >
Devices, Decisions: Glucose in the Critically ill
CAP
Today, January 2018, by Anne Ford
 Using
point-of-care glucose meters in critically ill
patients can feel like tiptoeing through a
regulatory minefield. Perhaps your preferred meter
hasn’t been cleared by the FDA for use in this
population. Or maybe you’re not sure which assay
performance requirements should be regulating the
performance of your meters. Or perhaps you’re still
trying to define “critically ill.” Using
point-of-care glucose meters in critically ill
patients can feel like tiptoeing through a
regulatory minefield. Perhaps your preferred meter
hasn’t been cleared by the FDA for use in this
population. Or maybe you’re not sure which assay
performance requirements should be regulating the
performance of your meters. Or perhaps you’re still
trying to define “critically ill.”
Recently
published studies have aimed to clear some of those
mines by evaluating the accuracy of glucose meter
results in ICU and non-ICU settings and by also
assessing meter performance in a clinical context
rather than a strictly analytical manner. Those
studies, the four options labs have, and a look at
the POC policy in place at Ohio State University
Wexner Medical Center were spotlighted at last
year’s AACC annual meeting in a session, “The Burden
of Proof for Point-of-Care Glucose Monitoring in
Critically Ill Patients,” presented by James H.
Nichols, PhD; Alison Woodworth, PhD; and Steven
Cotten, PhD.
While nursing tends to think that capillary samples
are easier than phlebotomy, Dr. Nichols said,
variations in operator technique mean there is ample
room for error. And getting an adequate reflection
of the patient’s physiology isn’t a given. What if
the patient is...
Read more >
Looking Beyond HbA1c Outcomes for Type 1 Diabetes
Leading
diabetes organizations release consensus definitions
for hypoglycemia, hyperglycemia, time in range,
diabetic ketoacidosis.
January 4,2018; CLN Stat
A consensus report by major diabetes organizations
includes standard definitions for outcomes in type 1
diabetes, including hypoglycemia, hyperglycemia,
time in range, and diabetic ketoacidosis.
After doing their homework on the clinical evidence,
major diabetes organizations issued a series of
priority outcomes for type 1 diabetes (T1D), looking
beyond the scope of hemoglobin A1c (HbA1c). HbA1c,
which assesses mean blood glucose measures over a
3-month period, is an important tool in diabetes
care management. However, the test is limited in
that it can’t capture short-term fluctuations in
blood glucose, exposure to hypoglycemia and
hyperglycemia, or the impact of blood glucose
variations on quality of life.
“Recent advances in type 1 diabetes technologies and
research have made it feasible to assess the efficacy
of therapies and technologies using a set of
outcomes beyond HbA1C and to refine definitions of
outcomes such as hypoglycemia. However, while
definitions for hypoglycemia in clinical care exist,
they are not standardized, causing inconsistency in
the definitions used in different research studies,”
according to a statement from the American Diabetes
Association (ADA). Read more >
HbA1c Shows its Mettle Predicting Diabetes Risk
CAP
Today, Anne Paxton, December 2017
The
longitudinal Framingham Heart Study, which first
identified the concept of risk factors and made
serum LDL cholesterol a household name, could help
increase the celebrity status of HbA1c, with the
Oct. 26 publication of a new study in Diabetes Care.
International and national organizations since 2010
have recognized HbA1c as a valid way to diagnose
abnormalities in glycemia and diabetes mellitus. But
there has been less consensus on its use as a screen
for elevated diabetes risk.
It has been shown that elevated HbA1c and elevated
fasting glucose are better at diabetes prediction
than fasting glucose alone. But is HbA1c associated
with incident diabetes independently, such that
HbA1c results can identify individuals with high
diabetes risk? That was the question addressed in
the Diabetes Care retrospective study “Prediction of
type 2 diabetes by hemoglobin A1c in two
community-based cohorts,” in which the authors
reviewed extensive data collected on subjects of the
Framingham Heart Study and the Atherosclerosis Risk
in Communities (ARIC) study (Leong A, et al. doi.org/10.2337/dc17-0607).
Based on that data... Read more >
Role of Medical Devices in the Data
Management Processes of the Modern Clinical
Laboratory
By
Shawn Hall, MLO, November 2017
As the modern clinical laboratory becomes more
connected, it becomes increasingly difficult to
efficiently exchange and manage data. This is
especially true with regard to interoperability,
where data is exchanged among several clinical
systems. Yet diagnostic laboratories have become
such an integral part of the connected healthcare
paradigm that methods for expanding their
scalability, improving performance, and managing
data are critical to achieving the core objectives
of meeting the needs of clinicians and patients.
Laboratories have long stressed efficiency, safety,
and quality in the management of diagnostic data;
however, the focus has primarily been on the
analytical phase. But the trends toward lab
automation and increasing testing require that
laboratories constantly adapt to ever-changing data
management requirements in all phases of the testing
process. This article addresses pre- and
post-analytical data management processes; discusses
challenges such as patient safety, complex
workflows, and reporting responsibilities; and
examines how data management features of analytical
medical devices can have a positive impact in
today’s connected clinical laboratory. Read more >
Point-of-Care Tests Help Manage Influenza
CLP,
By Patrick Murray, PhD, November 2017
This year’s flu
season requires clinical labs to take into account a
variety of new considerations, including FDA
reclassification of RIDTs.
Vaccines are the best defense against influenza, but
predicting when influenza will occur and which
strains will appear is a challenge (see Figure 1).
In 2009, a novel strain of influenza A arose during
the summer, and continued with significant disease
into the fall.1 In 2013, a new strain, H3N2, made
its debut and was responsible for the majority of
disease that year.1 This strain has been the
predominant virus in seasonal outbreaks for the past
3 years. Despite the fact that this strain is
covered under current vaccines and was once again
responsible for the majority of influenza cases
during the 2016–2017 flu season, however, the
prevalence of the strain led to particularly severe
outcomes for children and older adults. Read more >
Horizons in Point-of-Care Testing
CLP,
By Kate McLaughlin, PhD, and Donna Hochberg, PhD,
November 2017
POC testing is unlocking new markets for commercial
IVDs, but reaching potential new customers can be a
challenge.
Diagnostic testing that is capable of being
performed at the point of care holds the potential
to improve patient care by enabling faster clinical
decisions with small sample volumes. New instrument
platforms are addressing historic concerns over
quality and data integrity to allow a wider variety
of tests to be performed outside of the lab. At the
same time, new models of more-convenient healthcare
delivery, and the prospect of capturing additional
revenue by performing tests for which lab
proficiency testing and inspections are waived under
the terms of the Clinical Laboratory Improvement
Amendments of 1988 (CLIA), are together giving
physicians, pharmacists, and nurses more power to
shift testing volume to the point of care.
Still, commercialization of point-of-care (POC)
technologies remains... Read more >
Benefits and Bumps of Shifting to Beaker
By Anne
Paxton, CAP Today, November 2017
If they were
located in the Land of Oz, laboratories selecting a
laboratory information system might not have to make
a choice between full functionality and seamless
integration with their electronic medical record
system. They could just follow the helpful advice of
the Scarecrow to Dorothy at a crossroads: “Go both
ways.”
Down here in Dorothy’s Kansas, however, having to
weigh an LIS that is part of an enterprise wide
solution against a standalone LIS creates a classic
quandary for hospital laboratories: Follow one brick
road and you may have top-flight integration between
the LIS and EMR but possibly less LIS functionality.
Go down the other road and you may acquire a
best-of-breed LIS but risk stumbling on the
interface with the EMR. Increasing
numbers of hospital and health care system
laboratories, already operating in an Epic
environment for their EMR, are casting their lots
with integration by choosing Epic’s Beaker for their
LIS. As of August 2017, Epic had 375 installations
worldwide, 28 of them between August 2016 and August
2017... Read more >
From Many One A Case Study on
Standardizing Point of Care Testing
Instrumentation
Clinical Laboratory News, Bench Matters:
November 2017, Brenda Suh-Lailam, PhD,
DABCC, FACB
Point-of-care testing (POCT) goes a
long way toward helping institutions
improve patient care by returning
fast and reliable results near
patients, thereby enabling prompt
clinical interventions. POCT’s vital
role in patient management makes it
popular across clinical settings but
often with different device types
for the same test. Our institution
is no exception to this POCT device
creep, as at one point we had more
than four types of blood gas
analyzers, three table tops and one
hand-held. Realizing that this
confounded many of our quality and
efficiency aims, we undertook a
deliberate and durable process to
standardize these instruments... Read more >
Convincing Hospitals
that Glucose Management Matters
By Dan
Fleshler | Published on 23 October 2017
Patients'
blood glucose (BG) levels in many American hospitals
run dangerously high, but hospitals aren’t doing
nearly enough to address the problem.
Between 70% and 80% of patients with diabetes
experience hyperglycemia when they’re hospitalized
for critical illnesses or have cardiac surgery. And
about 30% of all inpatients experience high blood
sugars (>180 mg/dL). Even if you stay in the
hospital for just a few days, rising glucose levels
increase the mortality risk and the risk of eventual
kidney failure, poor healing, dehydration and other
problems. Meanwhile about 6% of hospital inpatients
experience potentially dangerous hypoglycemia (low
blood sugar) as well!
It doesn’t have to be this way. In this day and age
of continuous glucose monitoring (CGM) and closed
loop technology, hospital diabetes management has
the potential for a seismic shift -- if they choose
to adopt these newer innovations.
For example, recently on Oct. 18, the FDA approved a
first-of-its-kind CGM for surgical ICUs that can
monitor glucose levels and alert physicians and
hospital staff of any highs or lows. It's a sign of
the times, as this type of tech to monitor glucose
and dose insulin promises to improve patient health,
reduce hospital readmissions and cut health care
costs.
Yet only about 10% of Americans hospitals now use
these “e-Glycemic solutions,” says Linda Beneze, CEO
of Monarch Medical Technologies, which provides
high-tech glucose management systems to hospitals. Read more >
How to Choose a Quality Improvement Project
Q&A
with Michael Astion, MD, PhD, Editor-in-Chief, CLN
Patient Safety Focus
In
the October issue of CLN, a reader asked...
'I took a new
job as a supervisor in a medium-sized hospital lab.
The lab is in reasonably good shape with no emergent
problems, but my co-workers and I agree that certain
areas need improvement. I am confident I can do a
quality improvement (QI) project and have worked in
organizations that used different approaches—like
Lean, Six Sigma, and homegrown strategies—and feel I
will be able to fit into any system.
I know how to
choose metrics and incremental goals. But how do I
know which problems to work on first? It is
important to me that these first QI projects succeed
and are meaningful to the staff.'
For Dr. Michael
Astion's answer, click here...
Keeping Up with POCT Regulatory Compliance
MLO,
October 2017, By Connie Mardis
Today, hundreds of tests once considered too complex
for point-of-care testing (POCT) are routinely
performed outside the laboratory. Due to hospitals’
decentralized structure, laboratory testing is
performed on a multitude of POCT devices from
various manufacturers in many hospital wards,
critical care departments, clinics, and physician
offices. Typically, POC devices in a hospital can
include dozens of blood gas analyzers, urine
chemistry and cardiac marker systems, and handheld
coagulation instruments, as well as hundreds of
glucose devices. Perceived barriers to implementing
POCT have been attributed to accountability factors
such as quality control, adequate staff training,
and oversight for accreditation purposes. This
article will review accreditation requirements and
advances in open, vendor-neutral POCT data
management to facilitate billing capture, regulatory
compliance, and inspection preparedness.
Why POCT? Because of its convenience, timeliness, and
potential to improve patient outcomes, POCT’s
popularity continues to rise.1 Near-patient testing
increases the likelihood that healthcare
professionals and the patient will receive test
results faster, which may facilitate faster
diagnoses, more timely treatment interventions, and
improved patient compliance. For example... Read more >
Urinalysis Quality
Control at the Point-of-Care
MLO,
October 2017, By Brian Fernandez
The goal of any clinical diagnostic test procedure
is to provide critical information in a timely
manner so that appropriate actions may be taken,
ultimately improving patient outcomes. Point-of-care
testing (POCT) is a term that has come to describe a
multitude of rapid medical tests that can be
performed at or near the site of patient care. The
most compelling benefit of these tests is that, as
opposed to having to wait hours or days for results
to arrive from an outside laboratory, clinicians can
obtain the results immediately, allowing for
clinical management decisions to be made while the
patient is still at the care facility. While the
implementation of rapid diagnostic tests dates back
to ancient history (sweet-tasting urine was once
commonly used to diagnose diabetes mellitus), it was
not until the 1950s that these rapid diagnostic
methods gained any real predictive value.
Today, the popularity and demand for POCT are
increasing rapidly. TriMark Publications estimates
that the global market for POCT was $14.5 billion in
2016, and is expected to grow by seven percent over
the next five years.
Urinalysis dipsticks at the point-of-care Urinalysis using multi-analyte dipsticks is a
point-of-care test performed at any hospital,
clinical laboratory, doctor’s office, health clinic,
and nursing facility. Various iterations of these
tests have existed for decades, and they continue to
be among the most commonly performed tests of any
kind. Urinalysis dipsticks contain... Read more >
FDA Raises the Bar for Flu
Tests, Aiming for Better Testing and Better Outcomes
Clinical Lab Products, October 2017, By K.C. McGrath
FDA’s recent
regulatory reclassification of antigen-based rapid
influenza diagnostic tests (RIDTs) from Class I to
Class II was prompted by concerns about the tests’
performance during severe flu seasons, most notably
during the H1N1 influenza pandemic of 2009. The goal
of the reclassification is to improve point-of-care
influenza testing, in order to reduce misdiagnoses
and accelerate linkage to appropriate treatment.
The Rationale for Reclassification FDA’s
device classification system reflects the regulatory
controls needed to ensure that devices are safe and
effective for human use. There are three device
classifications based on risk: Class I, Class II,
and Class III. All three classes of devices must
satisfy basic requirements specified by FDA
(‘general controls’), such as proper packaging and
labeling. Read more >
The Journey to 100% Point-of-Care Connectivity
Clinical Laboratory News, October 2017, By
Christiane Nooney, MHA/MBA, MT(AMT)
At first glance, the need for centralized
connectivity of point-of-care (POC) instruments may
seem conceptually at odds with the primary benefit
these devices provide. Indeed, caregivers generate
results and may well have acted on them by the time
POC staff can view test data on their middleware
server. Nonetheless, the value of POC connectivity
has risen steadily in concert with the growing
importance of informatics in care delivery.
Connectivity not only facilitates dissemination of
clinical data to caregivers across the house but
also provides numerous advantages to laboratorians
under the broad heading of compliance management.
Unlike hospital laboratories... Read more >
The Role of Lab Automation in Reducing Diagnostic
Errors
Medical
Laboratory Observer, By Brad F. Tieman, September
2017
A recent study
reported that medical error is the third-leading
cause of death in the United States, just ahead of
respiratory illness and behind only cardiac disease
and cancer. More than 200,000 American deaths each
year are associated with preventable harm in
hospitals. In addition to putting patients at risk,
medical errors contribute to substantial avoidable
costs estimated to exceed $17 billion annually in
direct costs in the U.S. alone. Given that up to 70
percent of clinician decisions are influenced by
laboratory test results, there is a major role for
the clinical laboratory to play in reducing
avoidable medical error, enhancing patient safety,
and improving outcomes. Click here to download the Executive
Brief
Lab error and patient safety The ECRI Institute publishes an annual report on the
Top 10 Patient Safety concerns. In the most recent
report, two of the top ten, “test result reporting
and follow-up,” and “patient identification errors,”
are directly related to issues that can be addressed
by the clinical laboratory. Patient identification
errors and test result reporting are associated with
the pre-analytical and post-analytical stages of
clinical diagnostic testing. And while recent
studies have shown... Read more >
Bringing data analytics to bear on diabetes care
CAP
Today, September 2017, By Amy Carpenter Aquino
September 2017—Can data move the dial on diabetes?
That’s the thinking behind Roche Diabetes Care’s new
partnership with Accenture, and it’s how some labs
and health care systems are already driving diabetes
care to a whole new level.
“At Roche Diabetes Care, we want to create a leading
open digital diabetes ecosystem,” says Yan Beynon,
head of digital and health solutions. To do so, the
company will use Accenture’s existing Intelligent
Patient Platform to build a core data platform that
gathers “vital pieces of diabetes information,” he
says. The data will be categorized, analyzed, and
transformed into “powerful insights to support
improved therapy routines and outcomes.”
Within this ecosystem, Beynon says, blood glucose,
insulin, blood pressure, and cholesterol levels,
along with co-medication, physical activity, food
intake, and other information, will be collected and
analyzed and put into context. “Having all
therapy-relevant data in one place and the smart
algorithms to perform the analyses will help to
improve therapy adaptation and results,” he says. Read more >
CDC
Releases 2017 National Diabetes Statistics Report
The National Diabetes Statistics Report is a
periodic publication of the Centers for Disease
Control and Prevention (CDC) that provides updated
statistics about diabetes in the United States for a
scientific audience. It includes information on
prevalence and incidence of diabetes, prediabetes,
risk factors for complications, acute and long-term
complications, deaths, and costs. These data can
help focus efforts to prevent and control diabetes
across the United States. Click here for a copy of the report.
A Limitless Lab
By
Peter Koerte, PhD, Clinical Lab Products, on August
2017
With
connectivity, point-of-care testing is on the front
line when it comes to diagnosing and managing
chronic diseases
Across the globe, studies of healthcare delivery
systems have led researchers to the inescapable
realization that fragmented patient care is not a
sustainable way to manage patient health in the face
of growing economic pressures. The United States,
for example, spends more on healthcare than any
other high-income nation, yet it is well known that
Americans have a lower life expectancy and graver
health outcomes than residents of many other
countries. Such disparities can be attributed, in
part, to the failure of encounter-based medicine to
meet the growing demands of a population that is
heavily afflicted with chronic disease. In response
to such trends, healthcare delivery systems in the
United States and elsewhere are in the midst of... Read more >
Training Non-Laboratorians to Perform
POCT
By Peggy
A. Mann, MS, MT(ASCP), Clinical Laboratory News,
Ask the Expert: August 2017
Peggy A. Mann, MS, MT(ASCP), delves
into the challenges that
point-of-care coordinators (POCCs)
face when training non-laboratorians
to perform POC testing, from time
constraints to designing programs
suited for a diverse range of
healthcare staff positions. Read more >
Elizabeth Terry
Named 2017 POCC of the Year!
Elizabeth
Anne (Betty) Terry is part of a two person POCT team that
manages 1500 POCT users at the Kaiser Permanente
Medical Center in Oakland, California. The POCT
program encompasses a 349-bed, state-of-the-art
hospital and 6 medical office buildings.
Betty is a native
of Pennsylvania, raised in the San Francisco Bay
Area, received a BA in Bacteriology from the
University of California, Berkeley and completed the
Curriculum in Medical Technology from University of
California, San Francisco. During the course of her
career Betty has worked in all shifts and all areas
of the clinical lab. Read more >
“Off-Label” Use of Blood Glucose Monitoring Systems
in Critically ill Patients
MLO
letters to the Editor, June 22, 2017
The
article by Scott Isbell, PhD, DABCC, entitled
“Bedside blood glucose testing in critically ill
patients,” published in the April edition of MLO
[2017;49(4):8-12] summarized some of the current
issues related to testing capillary blood and the
“Off-Label” use of blood glucose monitoring systems
(BGMS) in critically ill patients. However, in our
view the article did not completely nor adequately
explain all the recent concerns about using BGMS in
critically ill patient care settings and the reasons
behind the publication of the new FDA guidance with
specific critical care accuracy criteria and
clearance requirements for manufacturers. More
specifically, the article did not address the
patient dangers associated with using blood glucose
meters not cleared for use in critically ill
patients, nor the regulatory risks to hospitals when
they use a meter not cleared for critically ill
patients in an “Off-Label” application. The concern about
the accuracy of BGMS used in critically ill patients
is related to deaths and serious adverse events
reported in the US FDA MAUDE database, as well as
in peer-reviewed medical journals. These reports and
publications represent the tip of the iceberg and
are still being reported for certain glucose meters
used routinely in hospital settings. Read more >
CDC data show C. diff infection rates are falling
after steady increase
Preliminary analysis of data from the CDC's Emerging
Infections Program showed that the rate of new
Clostridium difficile infections in hospitals and
nursing homes nationwide declined by 9% to 15% from
2011 to 2014, suggesting revised antibiotic use
guidelines and more aggressive cleaning standards
are working. C. difficile infection rates climbed
annually from 2000 to 2010, and in 2011 caused
almost 500,000 illnesses and killed about 29,000
people in the US. Read more >
CDC: Flu season moderate,
influenza H3N2 most dominant strain
CDC researchers reported that flu activity this
season was moderate, with influenza A (H3N2) being
the most dominant strain for most of the season. The
findings in the agency's Morbidity and Mortality
Weekly Report also showed that flu vaccines
distributed this season were tied to a 42% lower
overall risk of flu-related medical visits. Read more >
poctHUB Launches: Take It for a Spin
By Christopher
Fetters, Nextivity, May 2017
poctHUB
is a portal for professional POCT products that
includes the ability to read and write reviews, link
journal articles, browse product specifications and
search for your next great POCT product. poctHub is a
clearinghouse of product information which is
unbiased and normalized across products. Each
product will be rated by its users based on Clinical
Efficacy, Reliability, Ease-of-Use, Product Support,
and an Overall score. Viewers will be able to see
how many other hospitals have connected to each
product and how many have reviewed each product.
Scientific journal articles, case studies, and
product brochures can all be linked to each product
limiting the need to scour the web for product
information. poctHUB will put much of the
information you need, right at your fingertips. Using poctHUB is
free for anyone who directly or indirectly treats
clinical patients: POCCs, lab managers, medical
directors, respiratory, nursing, POL office
managers, etc. Take it for a spin today!
Untangling Glycaemia and Mortality in Critical Care
Vincent Uyttendaele, , Jennifer L. Dickson, Geoffrey
M. Shaw, Thomas Desaive and J. Geoffrey Chase Critical Care,
June 2017
Glycaemic control (GC) in the intensive care unit
(ICU) is a controversial subject . Whereas some
studies showed improved mortality with GC within a
tight or intermediate range, several others studies
and larger analyses did not reproduce these results.
Increased hypoglycaemia induced by the GC protocol,
patient variability and/or protocol compliance
further confounds results. The strong associations of blood glucose (BG) level
and/or variability with mortality have been used to
make a case for GC. The association of moderate or
severe hypoglycaemia with increased mortality
similarly indicates that improved control must be
achieved safely, despite high inter- and intra-
patient variability. The association of high times
in intermediate bands with reduced mortality would
indicate... Read more > | Online | PDF
Looking at POCT Through a New “Value” Lens
By Kim
Futrell, June 22, 2017, Medical Laboratory Observer
Advances in technology, combined with the value
focus of today’s healthcare system, are changing the
how, when, and where of laboratory testing. As part
of this shift, these trends are increasing the
demand for point-of-care testing (POCT) and
broadening the impact that POCT can make in improved
patient outcomes and cost savings. Improvements in
patient satisfaction also can be realized when
laboratory results are made available in real time
at the patients’ point of care. In order to utilize
POCT to its fullest, however, we have to learn to
look at POCT with a different perspective than in
the past—through a new “value” lens. We have to
carefully determine when and where POCT can have the
most benefit and implement IT solutions that ease
the complexity of POCT integration and oversight so
that the benefits are not overshadowed by the burden
of management and integration.
Read more >
Bringing POCT
to the Community
American
Pharmacists Association
The expansion of POCT services to
pharmacies increases patient access, but
barriers exist. According to CDC, 8
million people have undiagnosed
diabetes, 240,000 people have
undiagnosed HIV, and 800,000 people have
undiagnosed hepatitis C in the United
States.1 POCT—medical diagnostic testing
performed in close proximity to the
patient and outside traditional,
clinical laboratory settings—can
identify all three of these diseases.
The testing can be provided at primary
care clinics, community pharmacies,
paramedical vehicles, rural and remote
areas, and during times of natural
disasters or emergencies.
POCT offers many advantages toward
improving the quality of, access to, and
cost effectiveness of patient care. For
example... Read
more >
HbA1c Test May Improve Diabetes Detection
By Miriam E
Tucker, Medscape
HbA1c may be the most
effective method to identify patients with
undiagnosed prediabetes and diabetes, and
point-of-care testing further enhances that
screening ability in primary-care settings, new
research suggests. The findings were published
recently in the Annals of Family Medicine by Heather
P Whitley, PharmD, of Auburn University Harrison
School of Pharmacy, Montgomery, Alabama, and
colleagues.
"First, diabetes and prediabetes need to be on our
radar as possible diagnoses. In the United States,
where we have such a heavy prevalence of diabetes,
we need to be thoughtful and aggressive in
screening," Dr Whitley told Medscape Medical News.
And, for screening purposes, the data suggest that
HbA1c... Read
more >
Note: You must be a
Medscape Subscriber to view this article.
Subscriptions are free. From Micro-hospitals
to Mobile ERs...
New Models of
Healthcare Create Challenges and Opportunities for
Pathologists and Medical Laboratories
Dark Daily
New low-cost alternatives to emergency department
and hospital visits could require flexibility from
pathology groups and clinical laboratories to
provide the best quality care
In response to the rising cost of conventional
hospital services, innovative healthcare models such
as micro-hospitals, bedless hospitals, and mobile
and freestanding emergency rooms (ERs), are
attempting to lower costs while maintaining quality
of care by providing alternatives to traditional ER
visits and hospital stays.
This means new challenges and opportunities for
pathology groups and medical laboratories that can
adapt to the different needs of these new healthcare
delivery models. Each different care model will want
clinical lab testing services and the reporting of
lab test results to be handled in ways that enable
these providers to achieve improved patient
outcomes. Read more > Molecular-Based Point-of-Care Laboratory
Testing Will Revolutionize Healthcare,
But Practical Considerations Exist
Contagion Live -
Infectious Diseases Today, William Todd Penberthy,
PhD
The American Academy of Microbiology had a
point-of-care colloquium in October 2016 that
brought together clinical laboratory
microbiologists, physicians, investors, global
health experts, patient collection experts,
government agencies, foundations, and commercial
industry to discuss how to enact modern molecular
diagnostic protocols most effectively for
point-of-care (POC) testing settings.
Contagion® sat down with the chair of the colloquium
and a commentary-author with vast experience in POC
clinical trials; Melissa B. Miller, PhD, Professor
and Director of the Clinical Molecular Microbiology
Laboratory, University of North Carolina School of
Medicine, Chapel Hill and Robin Patel, MD, ASM
Microbe 2017 Co-Chair, Director, Infectious Diseases
Research Laboratory, Mayo Clinic.
Dr. Miller emphasized that one of the issues at the
POC colloquium was about changing paradigms. Read
more >
In Flu Season Management, POC Molecular to the Fore
Cap
Today, May 2017, By Anne Paxton
May 2017—Stacked against some of the nation’s
previous bouts with influenza—such as the 2014–15
season—the 2016–17 flu season didn’t break records
for drama. To be sure, every flu season is
different, and regional variation was prominent. In
Central Texas, some outbreaks appeared to start
later than usual, but the dominant viruses were the
same as last year’s—H1N1, H3N2, and influenza B—says
Bob Fader, PhD, chief of the virology and
microbiology laboratory at Baylor Scott & White
Health, Temple, Tex. The strains identified were a
good match with this year’s trivalent and
quadrivalent vaccine. Testing volume was up, as were
positive PCRs.
From her vantage point in the northeast, “I’d say
this season was about average,” says Donna M. Wolk,
MHA, PhD, D(ABMM), system director of clinical and
molecular microbiology for Geisinger Health System,
Danville, Pa. Read more >
POCT HbA1c Boosts Screening, Identification
of Prediabetes, Diabetes
May
2017, Clinical Laboratory News
A
comparison of point-of-care (POCT) HbA1c
testing versus standard diabetes
screening tests in family medicine
clinic patients found that POCT HbA1c
identified significantly more patients
with previously undiagnosed
hyperglycemia and prediabetes (Ann Fam
Med 2017;15:162–4). The authors also
determined that systematically screening
patients via POCT HbA1c “greatly
increases the chances for a screen to
occur.” Read more >
Point-of-Care or Clinical Lab INR for
Anticoagulation Monitoring: Which to Believe?
By
Stacy A. Johnson, MD, Clinical Laboratory News,
April 2017
68-year-old female with a history of hypertension,
diabetes mellitus, stroke, and atrial fibrillation
presents for routine follow-up at your hospital’s
anticoagulation clinic. The clinical pharmacist
checks her international normalized ratio (INR) with
a point-of-care (POC) device to monitor her
anticoagulant therapy (warfarin).
The POC INR result is elevated to 4.0, which is
above the recommended INR goal range of 2.0–3.0
based on her clinical indication of atrial
fibrillation. The pharmacist enters the POC INR
result into the patient’s electronic medical record
and discovers she had an INR obtained earlier that
same day, along with a basic metabolic panel and
complete blood count ordered by her primary care
physician. The clinical lab (CL) INR result was 2.9,
and obtained just 90 minutes earlier. All other test
results were normal.
The patient says...... Read more >
Bedside Blood Glucose Testing
in Critically Ill Patients
By
T. Scott Isbell, MLO, April 2017
This
month's issue of MLO has a really good, continuing
education, article on how studies have demonstrated
that the practice of hospital bedside blood glucose
testing is a necessary and effective means of
managing and monitoring glycemic control. Protocols
vary by institution, but there is general consensus
among providers that this process is an essential
component of patient care. However, the use of
handheld blood glucose meters within some critically
ill patient populations has resulted in varying
degrees of confusion about off-label use and
potential discrepancies in results.
LEARNING OBJECTIVES
-
Define what
constitutes a critically ill patient population
and discuss the use of handheld blood glucose
monitors in critically ill populations.
-
Discuss
agencies that regulate off-label device use and
identify the guidelines that laboratories must
adhere to, in order to be compliant with
off-label device use.
-
Recognize the
characteristics of diabetes statistics as the
relate to healthcare and morbidity.
-
List testing
methods for diagnosing and monitoring diabetes
and define the limitations with each method.
Read more >
For more on diabetes/glucose testing, check out
the April MLO Digital Edition.
Management of Inpatient Hyperglycemia and Diabetes in Older Adults
Diabetes Care 2017;40:509–517 | DOI:
10.2337/dc16-0989
Adults aged 65 years and older are the fastest
growing segment of the U.S. population, and their
number is expected to double to 89 million between
2010 and 2050. The prevalence of diabetes in
hospitalized adults aged 65–75 years and over 80
years of age has been estimated to be 20% and 40%,
respectively. Similar to general populations, the
presence of hyperglycemia and diabetes in elderly
patients is associated with increased risk of
hospital complications, longer length of stay, and
increased mortality compared with subjects with
normoglycemia. Clinical guidelines recommend target
blood glucose between 140 and 180 mg/dL (7.8 and 10
mmol/L) for most patients in the intensive care unit
(ICU). A similar blood glucose target is recommended
for patients in non- ICU settings; however... Read more
HbA1c in CVD Treatment: Farewell to One Size Fits All
By Anne
Paxton, CAP Today, March 2017
Anchor. Central pillar. Cornerstone. It would be
hard to find a weighty synonym for “linchpin” that
hasn’t been used to describe HbA1c’s role in
diabetes diagnosis and management since 2010, when
the assay was recognized by key standard-setting
organizations as the equal of fasting glucose and
oral glucose tolerance testing in diabetes and
prediabetes testing. But recognition of the complex nature of the
relationship between HbA1c and diabetes-related
complications has influenced and modified HbA1c’s
clinical use as the test evolves. A new review
article by experts in the field outlines how use of
the HbA1c test in cardiovascular disease treatment
and prevention is trending toward a more
patient-centered approach as the assay’s intricacies
are explored. Read more >
Diabetes Decision
Time: Proficiency testing
hurdle slows use of POC HbA1c
tests
By:
Deborah Levenson, March 2017, Clinical Laboratory
News
More than 29 million Americans—about 9% of the U.S.
population—have diabetes, according to the American
Diabetes Association (ADA). Racial and ethnic
minority groups have higher rates of the disease,
which, when not managed effectively, leads to
debilitating complications like cardiovascular
disease, kidney disease, stroke, and blindness. As
it is, however, some patients are well down the road
to developing these sequelae before being diagnosed
formally with diabetes. Since point-of-care (POC)
tests that measure HbA1c are well-established tools
for monitoring and managing long-term glycemic
control, some healthcare professionals believe using
them for diagnosis would catch individuals earlier
in the diabetes disease process, enabling timelier
treatments and better outcomes. More >
Advances in POCT technologies outpace regulatory and
accreditation requirements
Jeffrey A. DuBois, MLO, February 2017
Long after their deaths, two famous scientists
continue to challenge us with their words. “Knowing
is not enough; we must apply. Being willing is not
enough; we must do,” said Leonardo da Vinci, Italian
artist, scientist, and inventor. “The true sign of
intelligence is not knowledge but imagination,”
spoke German-born theoretical physicist Albert
Einstein. Their pioneering work has influenced all
areas of science and, perhaps, even science fiction.
The imaginary tricorder in the Star Trek series may
represent the ultimate goal of integrated
point-of-care diagnostics, but it remains a
fictional object. However, the Internet of
individual care (and with it, the creation of high
volumes of clinical data), where sensors, tests, and
wearable devices have moved out of the laboratory
and clinic directly into our lives for
self-management and remote monitoring, has already
begun and presents significant challenges to
providers, regulators, and accreditation agencies
alike. More >
IQCP: The Critical First-Year Findings
By
Irwin Rothenberg, MBA, MS, MLS(ASCP), Advance for
Administrators of the Lab, February 2017
A
year ago, laboratory journals, professional meetings
and in-house planning were all about the pending
deadline for implementation of the new CMS quality
control option, the Individualized Quality Control
Plan (IQCP), which was replacing the Equivalent
Quality Control (EQC) testing already in place. With the January
1, 2017 implementation date rapidly approaching, many laboratorians had already begun performing their
risk assessments, and making revisions to their
existing QC Plans (as needed). Laboratory quality
assessment schedules were revised to include QAs for
the new IQCPs.
Whether or not IQCP is judged effective at actually
improving the quality of the testing performed
depends on how well the implementation process is
carried out, along with the subsequent quality
assessments performed. More >
How did your lab do with POCT?
Patrick
Murray, MLO, February 2017
The end of flu
season is in view
At this point in the year, most healthcare providers
are seeing a steady wave of patients with flu-like
symptoms. They may be struggling to pinpoint
underlying causes and identify the appropriate
treatment in a timely manner. Flu and respiratory
syncytial virus (RSV) have overlapping peak
infection seasons, making it difficult to
distinguish the two clinically. Group A
streptococcus is also common now.
We’ve likely seen the peak of flu season, and RSV
may be starting its wind-down as well, so this is a
good time to begin reflecting on how point-of-care
testing (POCT) in your institution affected
performance in managing winter respiratory tract
infections. Upon reflection, how might your POCT
strategy be improved for the next flu season? Here
are some considerations for lab managers, which may
help with planning for 2017-2018.
Effect on
overuse of antibiotics
An incorrect diagnosis or inaccurate test result may
point providers to the wrong treatment, exacerbating
the overuse of antibiotics. More than 25 percent of
antibiotics are prescribed for conditions that don’t
warrant them.3 Out of 97 million annual office
visits among adults in the United States between
2007 and 2009 that resulted in an antibiotic
prescription, 41 percent were for a respiratory
condition, the most common out of seven categories. More >
Accriva Diagnostics Acquired by Werfen and Instrumentation Laboratory
Accriva
Diagnostics, a Warburg Pincus portfolio company,
announced today the definitive agreement with Werfen,
a privately held medical diagnostics firm
headquartered in Barcelona, Spain, and its
subsidiary Instrumentation Laboratory (IL)
headquartered in Bedford, MA, whereby Werfen and IL
have acquired all shares of Accriva. The transaction
was successfully closed on January 19, 2017.
The Accriva portfolio, including globally recognized
point-of-care (POC) diagnostic products for
coagulation and anti-platelet therapy response, will
allow IL to establish a market-leading position in
hospital-based POC Hemostasis testing, expand its
position in POC Critical Care testing and complement
its leadership in the Hemostasis Laboratory segment.
Accriva Website | Accriva Press Release | Werfen/IL Press Release
Is improving access for patients equaling loss of
critical quality oversight?
Advance
for Adminstrators of the Lab, At the Bedside, By
Peter Koerte on January 2017
Diagnostic testing traditionally performed in
healthcare settings such as hospitals and reference
laboratories is increasingly expanding beyond the
brick-and-mortar boundaries of which we’ve grown
accustomed. Today, point-of-care testing (POCT), or
testing conducted outside the laboratory, is quickly
evolving to help expedite patient care and clinical
decision-making.
There are key benefits to the point-of-care testing
approach. The first pertains to response time and
its effect on patient care. Critical Stat tests can
be processed more quickly, expediting patient care
for emergency situations such as suspected venous
thromboembolism. More >
FDA Releases Two Final Guidance Documents
on Blood Glucose Monitors
Clinical Laboratory News, December 2016
The
Food and Drug Administration (FDA) has released the
final versions of two guidance documents, “Blood
Glucose Monitoring Test Systems for Prescription
Point-of-Care Use” and “Self-Monitoring Blood
Glucose Test Systems for Over-the-Counter Use.”
These documents describe the studies and criteria
that manufacturers should submit when seeking
clearance for blood glucose monitors designed for
use by healthcare professionals and patients at
home, respectively. More > The complete
versions of both guidance documents are available at www.fda.gov
Point-of-Care Testing: The Great Boom Ahead
Kim Futrell, MT (ASCP),
Products Marketing Manager, Orchard
Software Corporation | Cap
Today Online
White Paper
features POC testing management and integration
A
new white paper from Orchard Software titled
“Point-of-care Testing: The Great Boom Ahead”
discusses the history of POC testing connectivity
and shares specific case study savings and
improvements associated with the adoption of a POC
testing connectivity and management solution.
The value-focused environment of today’s healthcare
is increasing the demand for POCT; and not just POCT,
but rapid, accurate, and integrated POCT results.
Improvements in patient outcomes and satisfaction
can be realized when laboratory results are made
available in real-time at the patients’
point-of-care. Yet, POCT tends to be siloed and
often is not managed by the lab. To meet the
evolving needs of healthcare, laboratories need to
actively manage POCT. To do so effectively... More
>
Laboratory Management of POCT
Thorough
evaluation and stewardship by the laboratory are
necessary before and during implementation.
By Kim
Futrell, BS, MT(ASCP), 30 NOVEMBER 2016 • ADVANCE
/LABORATORY • WWW.ADVANCEWEB.COM
Thorough
evaluation and stewardship by the laboratory are
necessary before and during implementation.
The demand for point-of-care testing (POCT) is
increasing in response to the value-shift in
healthcare and advancements in technology. A number
of factors are converging to promote the value of
POCT (e.g., increases in infectious diseases;
increases in lifestyle diseases, such as cardiac
diseases and diabetes; increased patient desire to
use home-based POC devices; and technological
advancements creating faster and easier-to-use
devices). More >
CAP to Discontinue WBG WG2 Surveys
The College of
American Pathologists’ (CAP) Laboratory
Accreditation Program (LAP) will no longer require
enrollment and participation in proficiency testing
(PT) for waived whole blood glucose on glucose
meters (ie, WB2/WBG) and waived whole blood Protime/INR
(ie, WP10) beginning with the 2017 PT program year.
Laboratories will be required to perform alternative
performance assessment for these analytes. To read the letter from CAP, click here. If you have
questions regarding this change, please contact a PT
Compliance representative at 1-800-323-4040 ext.
6052 or 1-847-832-7000 ext. 6052. To learn more
about CAP Quality Crosscheck products, please visit the CAP online store (please note,
the whole blood glucose Quality Cross Check product
will not be available until the 2017 ordering
information is posted) or contact CAP Customer
Service at 1-800-323-4040 (Option 1).
Addressing Antibiotic Resistance with Molecular
Diagnostics
New methods promise
faster and more accurate detection of MDROs
By
Elena V. Grigorenko, PhD, and Donald R. Stalons,
PhD, D(ABMM), MPH, CLP, October 2016
Antibiotics are marvels of modern medicine that have
helped healthcare professionals fight infections
caused by bacteria for the past 70 years. However,
there is concern for the current use and future
benefits of antibiotics. According to the World
Health Organization (WHO), widespread inappropriate
use of antibiotics has created global resistance
that may soon drive every nation into a
post-antibiotic era. More >
Rapid point-of-care assays for influenza testing
By:
Norman Moore, October 2016, MLO
The estimated overall vaccine effectiveness rate for
the 2015-2016 flu vaccine was below 50 percent,
underscoring the importance of accurate and timely
diagnosis for improving patient outcomes and
reducing the public health impact of this
potentially deadly illness.
Clinical guidelines from the U.S. Centers for
Disease Control and Prevention (CDC) and other
expert groups recommend initiating antiviral
treatment within 48 hours of onset.1 Yet many of the
influenza assays widely used today do not realize
the full potential of diagnosis because they do not
provide highly accurate results quickly enough for
clinicians to make informed treatment decisions
while the patient is still under their care. More >
CMS: Nurses Can Perform
High Complexity Tests
By Glen
McDaniel, Advance Newsmagazines - www.advanceweb.com
The
Centers for Medicare and Medicaid Services (CMS)
recently published a memo that it sent to CLIA
inspectors on how to interpret educational
requirements. CLIA had always specified the minimum
educational requirements for individuals performing
laboratory tests. In recent years
there has been quite a strong lobby from nursing to
recognize a nursing degree as a biological science
degree, having the requisite credit hours of
biology, chemistry etc. Those of us with oversight
for point of care testing (POCT) had also been
unsure as to whether nurses could perform non-waived
tests and maybe even fully manage a POCT program
where non-waived tests were utilized. Now CMS has
weighed in definitively by saying that, yes, a
nursing degree is a science degree making nurses
qualified to perform non-waived tests. A careful
reading of the CLIA regulations would suggest that
if that is true, then nurses may in fact be allowed
to even direct laboratory testing. Read more >
Point-of-Care Hemoglobin Testing: Methods and Relevance to Combat Anemia
By:
Katja Lemburg, Medical Laboratory Observer,
September 2016
Anemia is a
condition that causes a high degree of personal
disability but, historically, has lacked adequate
resourcing in many public health systems. This
situation is even less understandable when you
consider that the main diagnostic, hemoglobin
testing, is one of the most commonly used
point-of-care (POC) tests, and one of the easiest to
perform.
POC hemoglobin testing is often needed in settings
where the use of a benchtop laboratory hematology
analyzer is not practical. It is ideal for use in
settings where resources are poor, or there is a
need for mobility and simplicity in field use, or
where turnaround time (TAT) for the test result
needs to be short, as in acute clinical situations. Read more >
Let’s Close the Knowledge Gap
CAP
Today, From the President's Desk, August 2016
Most
of us have heard the laboratory described as a black
box where specimens are exchanged for information
and diagnoses. This tells me that we work beside
some highly skilled people who don’t know what we do
and that the knowledge gap makes them uncomfortable
enough to joke about it. This incomplete
understanding of what takes place within the
laboratory has meaningful consequences in multiple
contexts.
I am certain you
will be asked (often by someone well into the
process of creating a budget) to quantify your value
to the institution. Because this is virtually
inevitable, we should anticipate it and formulate a
succinct response. For example, one might say that
we know how to ask the right questions, to work with
complex systems, or to keep stuff from blowing up.
Ideally, we can transition from there to the real
answer: Our value lies more in how we think than in
what we do. Read more >
Not fit to test: battling high hemolysis rates in
the ED
By Anne
Ford, CAP Today, August 2016
Poverty, unemployment, crime, dropout rates: In some
categories, no community wants to be No. 1. And in
some categories, no hospital wants to be No. 1
either. High on that list: hemolysis.
“Hemolysis is a big issue,” Michael Phelan, MD, said
at the Executive War College meeting this spring. In
fact, “it’s the leading cause of unsuitable
specimens” at the Cleveland Clinic’s main campus,
where Dr. Phelan is an emergency medicine physician.
As he discovered at the start of a CDC-funded
project to study and reduce hemolysis at his
hospital between 2014 and 2015, his emergency
medicine department led all other departments in
hemolysis rates. Over the course of one week... Read more >
2016 AACC POCC Forum
Highlight
Effective communication skills when discussing an
overdue PPM competency assessment with a physician
The 2016 Point of
Care Forum topic at the AACC Annual Conference in
Philadelphia
was ‘Leadership Communication for the POCC:
Overcoming the Barriers of Productive Communication’,
presented by Rick Import of Whitehat Communications.
Among the forum highlights was a role play session
'acted' by a panel of POCCs. One of the role play
dialogs that drew a lot of attention from the
audience was between a POCC and physician on the
subject of PPM competency. Many in attendance
requested that it be made available for reference in
their own settings.
Here is that dialog. We hope it helps and want to thank Marcia Zucker,
PhD for providing the segment of the dialog that
POCC’s will find so valuable when discussing PPM
competency with physicians.
Note: Many
more POCT highlights from AACC will be available on
this website and through the PointofCare.net
eNewsletter in the coming weeks.
|

 Quality is the mantra of medical laboratory professionals, but what is quality? What does it really mean? Why does it matter? Isn’t quality control (QC) good enough? Isn’t it the same thing as quality assurance (QA)? If we run quality control samples, why do we bother with external proficiency testing (EPT)? Isn’t plotting QC results on graphs good enough? What is the point of spending all this time and money on quality? Is it really worth it?
Quality is the mantra of medical laboratory professionals, but what is quality? What does it really mean? Why does it matter? Isn’t quality control (QC) good enough? Isn’t it the same thing as quality assurance (QA)? If we run quality control samples, why do we bother with external proficiency testing (EPT)? Isn’t plotting QC results on graphs good enough? What is the point of spending all this time and money on quality? Is it really worth it? 
 Clinical Laboratory News / July/August 2023
Clinical Laboratory News / July/August 2023 Patients are more comfortable with finger pricks versus a venous draw, which is strengthening the popularity of point-of-care testing across the globe, shared Prof. Rajiv Erasmus, Head of the Department of Chemical Pathology at the University of Stellenbosch in South Africa, at his session, “Connectivity strategies in managing Point of Care services” that took place on Day two of the Medlab Middle East Congress 2023.
Patients are more comfortable with finger pricks versus a venous draw, which is strengthening the popularity of point-of-care testing across the globe, shared Prof. Rajiv Erasmus, Head of the Department of Chemical Pathology at the University of Stellenbosch in South Africa, at his session, “Connectivity strategies in managing Point of Care services” that took place on Day two of the Medlab Middle East Congress 2023. Kathleen is the POCT Manager at Tricore, responsible for general operation of assigned Point-of-Care Testing (POCT) at TriCore Sites: 15 hospitals and 140+ clinics. She manages the department and coordinates activities with other areas of the organization and with internal and external customers to ensure that quality standards are met.
Kathleen is the POCT Manager at Tricore, responsible for general operation of assigned Point-of-Care Testing (POCT) at TriCore Sites: 15 hospitals and 140+ clinics. She manages the department and coordinates activities with other areas of the organization and with internal and external customers to ensure that quality standards are met.  Rick founded Whitehat Communications in 2008 to fill an educational need for Point-of-Care Coordinators (POCCs).
Rick founded Whitehat Communications in 2008 to fill an educational need for Point-of-Care Coordinators (POCCs).  Whole
blood viscoelastic testing (VET) for
perioperative bleeding management is
systematically increasing in clinical
use and is approaching the level of
standard of care for many clinical
settings such as cardiovascular surgery,
liver transplantation, trauma, and
obstetric hemorrhage. Conventional
coagulation testing has proven to be
inadequate for directing therapeutic
intervention in these critical settings.
Whole
blood viscoelastic testing (VET) for
perioperative bleeding management is
systematically increasing in clinical
use and is approaching the level of
standard of care for many clinical
settings such as cardiovascular surgery,
liver transplantation, trauma, and
obstetric hemorrhage. Conventional
coagulation testing has proven to be
inadequate for directing therapeutic
intervention in these critical settings. Using
point-of-care glucose meters in critically ill
patients can feel like tiptoeing through a
regulatory minefield. Perhaps your preferred meter
hasn’t been cleared by the FDA for use in this
population. Or maybe you’re not sure which assay
performance requirements should be regulating the
performance of your meters. Or perhaps you’re still
trying to define “critically ill.”
Using
point-of-care glucose meters in critically ill
patients can feel like tiptoeing through a
regulatory minefield. Perhaps your preferred meter
hasn’t been cleared by the FDA for use in this
population. Or maybe you’re not sure which assay
performance requirements should be regulating the
performance of your meters. Or perhaps you’re still
trying to define “critically ill.”