|
Clinical
Innovations
747 West 4170
South
Murray, UT
84123
Phone:
(888)268-6222
Fax:
(802)266-7373
Email:
mail@clinicalinnovations.com |

www.clinicalinnovations.com |
|
|
COMPANY DESCRIPTION: |
|
Clinical Innovations develops,
manufactures and markets state-of-the-art medical devices that improve
healthcare outcomes for clinicians and their patients worldwide. With
particular emphasis on healthcare solutions for women and their infants,
Clinical Innovations employs high quality standards in every aspect of
business to make advancements in technology and bring innovative ideas
to life. |
|
|
ROM Plus Fetal
Membranes Rupture Test: |
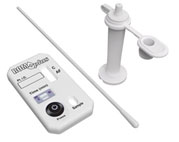 ROM
Plus Fetal Membranes Rupture Test is a rapid, qualitative test for the
in vitro detection of amniotic fluid in vaginal secretions of pregnant
women. It utilizes a combination of monoclonal and polyclonal antibodies
to detect alpha-fetoprotein (AFP) and placental protein 12 (PP12, or
insulin growth factor binding protein-1) found in amniotic fluid.
Testing for two protein markers using the monoclonal and polyclonal
antibody combination increases ROM
Plus Fetal Membranes Rupture Test is a rapid, qualitative test for the
in vitro detection of amniotic fluid in vaginal secretions of pregnant
women. It utilizes a combination of monoclonal and polyclonal antibodies
to detect alpha-fetoprotein (AFP) and placental protein 12 (PP12, or
insulin growth factor binding protein-1) found in amniotic fluid.
Testing for two protein markers using the monoclonal and polyclonal
antibody combination increases
ROM Plus' sensitivity and improves
diagnosis, particularly of PPROM. Housed in a convenient test cassette,
ROM Plus quickly and accurately provides test results essential to
optimizing perinatal outcomes. |
|
Features:
-
Two Protein Markers:
Testing for both AFP and PP12 improves diagnostic accuracy.
-
Lateral Flow Technology:
Latest in lateral flow test strip technology makes performing the
test and reading results easy.
-
On-Demand, Rapid Test:
No need for a speculum. Using at prescription point-of-care sites
produces immediate results.
-
Convenient Cassette:
Cassette contains test strip, built-in timer and patient ID tracker
for convenience, portability and quality control.
-
Spill-Resistant Vial/ Scored
Swab:
Preventing spills enables a clean test procedure and reduces need
for re-sampling. Keeping the swab tip in the vial reduces mess.
|
Test Kit Requirements:
-
CLIA Classification:
Moderately complex
-
Analytes performed on this
device:
Identifies presence of AFP (alphafetoprotein) and PP12 (placental
protein 12, insulin growth factor binding protein)
-
CPT Code:
In process
-
Test methodology:
Rapid Immunoassay
-
Sample volume:
Threshold 5ng/mL
-
Time to result:
5-20 minutes
-
Units in which the test is
reported:
Quantitative: ± result
-
MSDS's associated with the use
of this product:
N/A
-
Instrument requirements:
N/A
-
Software information
requirements:
N/A
|
|