|
Conworx Data Solutions
America, Inc.
100 Cummings Center, Suite
445-C
Beverly, MA 01915
Sales Support Phone Number:
855-426-6979
Sales Support E-Mail:
Sales@us-conworx.com |

www.conworx.com
|
|
CORPORATE DESCRIPTION:
Conworx is a global leader in
Point of Care and medical device connectivity and data management
software solutions. The company was founded in 1999 and has offices in
the United States, Germany, United Kingdom, and France. United States
Operations is located in Beverly, Massachusetts. Conworx’s data
management solutions are used in over 1700 hospitals in 20 countries.
Over 100,000 medical and laboratory devices have been connected over the
past 15 years. In addition, Conworx delivers innovative technology
solutions for independent labs, physician networks, clinics, and at-home
monitoring.
|
|
PRODUCTS:
UniPOC Point-of-Care Data Manager
is marketed in the United States, Canada, and Latin America. UniPOC’s robust
and scalable software components are designed to meet the POC data
management needs of large health networks to small healthcare facilities. In
addition, UniPOC offers an open architecture, meaning easier management of
operator compliance in terms of certifications, quality, and education.
I
Software screens
|
|
Version:
|
Download Capability:
- Interfaced to 100+
POC devices – devices may download to UniPOC via network PC,
Digi/Lantronix, Direct, and supports wireless
|
|
Operating System Capability:
-
32 / 64 Bit OS
Compatibility – UniPOC will work in 64bit on Windows Server
2008 R2 and Windows Server 2012.
-
Windows Server 2003 SP2 (32 bit)
-
Windows Server 2008 (32 bit)
-
Windows Server 2008 R2
-
Windows Server 2012
-
SQL
Server 2008 (32bit)
|
Administrative Features:
-
Administrative Overview
-
Organization Setup
-
Facility Setup
-
Department Setup
-
Location Setup
-
Facility Settings
-
My
Profile
-
Database Screens
-
Database System Status
-
Panels Screens
Back to top |
|
User Features:
|
QC Features:
Inventory Control Features:
-
Audit Trail Log
-
Tracking Log
-
Reagent Usage Report
Back to top |
|
Instrument Management
Features:
-
Instruments Overview
-
Moving, Renaming, and
Deleting Instruments
-
Instrument Configuration
-
Instrument Comment Codes
-
Instrument Port
Assignment
-
Tracking Log
-
Upload Log
Install Base:
Export Capability:
-
UniPOC supports importing
and exporting of data and reports
-
Imports Learning
Management Systems
-
Provides Secure E-mail
Integration
-
Supports Active Directory
LIS Interface Capability:
-
Supports Scripted and HL7
interfaces
-
Uni-Directional /
Bi-Directional (per device type)
-
Solicited – Unsolicited
Interfaces
-
Interface relationships
will all major LIS / EMR vendors
|
Reporting Features:
-
Audit Trail
-
Comment Codes
-
Control Data Stats by
Instrument
-
Control Data Stats by
Reagent
-
Data Listing
-
Glucose Meter Nurse
Manager Report
-
Glucose Meter QI Report
-
Instrument Config Report
-
Instrument Error Report
-
Instrument Workload Stats
-
Levey-Jennings Graft
-
LJ Graft by Reagent
-
Linearity Graft
-
Operator Certification
Expiration Report
-
Operator Competency
Report
-
Patient Outlier Report
-
Proficiency Data Listing
Report
-
QC Outlier Report
-
I-Stat Data Listing
-
I-Stat Instrument Event
-
Quality Check Codes
-
Quality Check Codes by
Operator
-
·Reagent Usage
Back to top |
|
Our processes are based on
the standards FDA 21CFR PART 820 and DIN ISO 9001:2008
Back to top |
|
SOFTWARE SCREENS: |
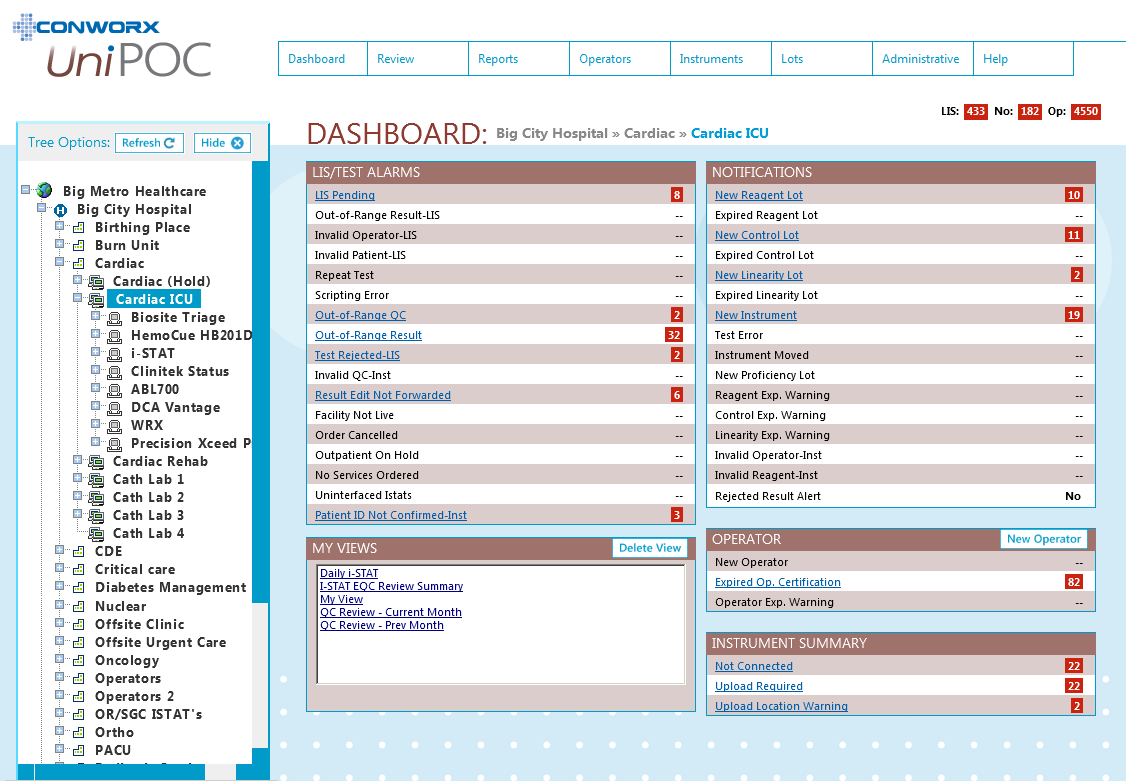
Back to top |
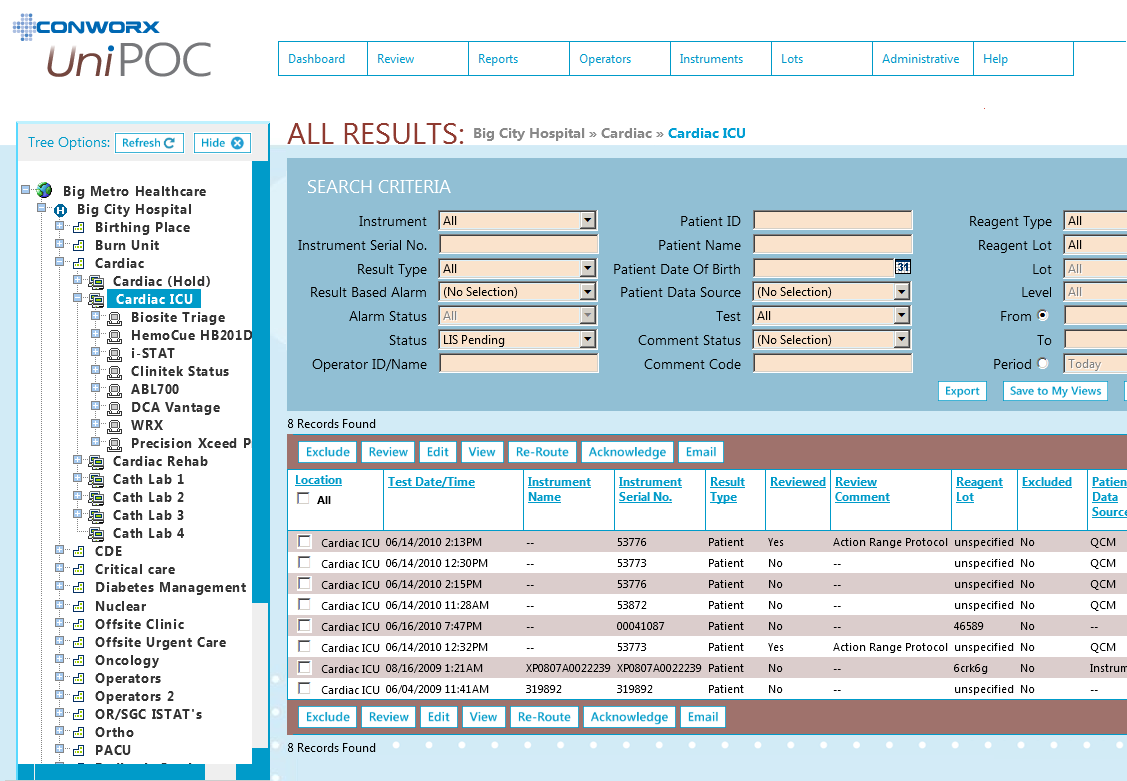
Back to top |
|
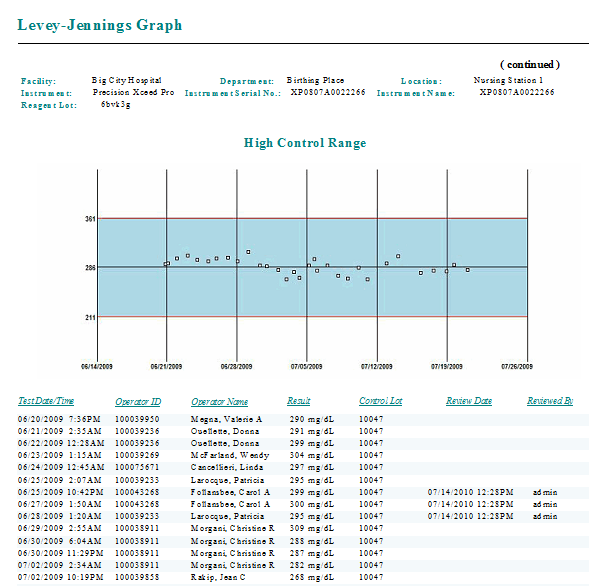
Back to top |
|
|